Copper-Modified Polymeric Membranes for Water Treatment: A Comprehensive Review
- PMID: 33525631
- PMCID: PMC7911616
- DOI: 10.3390/membranes11020093
Copper-Modified Polymeric Membranes for Water Treatment: A Comprehensive Review
Abstract
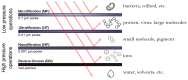
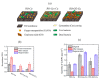
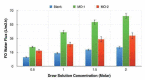
In the last decades, the incorporation of copper in polymeric membranes for water treatment has received greater attention, as an innovative potential solution against biofouling formation on membranes, as well as, by its ability to improve other relevant membrane properties. Copper has attractive characteristics: excellent antimicrobial activity, high natural abundance, low cost and the existence of multiple cost-effective synthesis routes for obtaining copper-based materials with tunable characteristics, which favor their incorporation into polymeric membranes. This study presents a comprehensive analysis of the progress made in the area regarding modified membranes for water treatment when incorporating copper. The notable use of copper materials (metallic and oxide nanoparticles, salts, composites, metal-polymer complexes, coordination polymers) for modifying microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), forward osmosis (FO) and reverse osmosis (RO) membranes have been identified. Antibacterial and anti-fouling effect, hydrophilicity increase, improvements of the water flux, the rejection of compounds capacity and structural membrane parameters and the reduction of concentration polarization phenomena are some outstanding properties that improved. Moreover, the study acknowledges different membrane modification approaches to incorporate copper, such as, the incorporation during the membrane synthesis process (immobilization in polymer and phase inversion) or its surface modification using physical (coating, layer by layer assembly and electrospinning) and chemical (grafting, one-pot chelating, co-deposition and mussel-inspired PDA) surface modification techniques. Thus, the advantages and limitations of these modifications and their methods with insights towards a possible industrial applicability are presented. Furthermore, when copper was incorporated into membrane matrices, the study identified relevant detrimental consequences with potential to be solved, such as formation of defects, pore block, and nanoparticles agglomeration during their fabrication. Among others, the low modification stability, the uncontrolled copper ion releasing or leaching of incorporated copper material are also identified concerns. Thus, this article offers modification strategies that allow an effective copper incorporation on these polymeric membranes and solve these hinders. The article finishes with some claims about scaling up the implementation process, including long-term performance under real conditions, feasibility of production at large scale, and assessment of environmental impact.
Keywords: biofouling; copper nanomaterials; nanocomposites; polymeric membranes; water treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Mussel-Inspired Architecture of High-Flux Loose Nanofiltration Membrane Functionalized with Antibacterial Reduced Graphene Oxide-Copper Nanocomposites.ACS Appl Mater Interfaces. 2017 Aug 30;9(34):28990-29001. doi: 10.1021/acsami.7b05930. Epub 2017 Aug 16. ACS Appl Mater Interfaces. 2017. PMID: 28767226
-
Progress for Co-Incorporation of Polydopamine and Nanoparticles for Improving Membranes Performance.Membranes (Basel). 2022 Jun 30;12(7):675. doi: 10.3390/membranes12070675. Membranes (Basel). 2022. PMID: 35877880 Free PMC article. Review.
-
A Comprehensive Review of Performance of Polyacrylonitrile-Based Membranes for Forward Osmosis Water Separation and Purification Process.Membranes (Basel). 2023 Nov 3;13(11):872. doi: 10.3390/membranes13110872. Membranes (Basel). 2023. PMID: 37999358 Free PMC article. Review.
-
Insights into the impact of polydopamine modification on permeability and anti-fouling performance of forward osmosis membrane.Chemosphere. 2022 Mar;291(Pt 1):132744. doi: 10.1016/j.chemosphere.2021.132744. Epub 2021 Oct 29. Chemosphere. 2022. PMID: 34743795
-
Performance improvement and application of copper-based nanomaterials in membrane technology for water treatment: A review.J Environ Manage. 2024 Nov;370:122755. doi: 10.1016/j.jenvman.2024.122755. Epub 2024 Oct 8. J Environ Manage. 2024. PMID: 39378812 Review.
Cited by
-
Chelating Silicone Dendrons: Trying to Impact Organisms by Disrupting Ions at Interfaces.Molecules. 2022 Mar 14;27(6):1869. doi: 10.3390/molecules27061869. Molecules. 2022. PMID: 35335233 Free PMC article.
-
Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration.Polymers (Basel). 2023 Aug 16;15(16):3421. doi: 10.3390/polym15163421. Polymers (Basel). 2023. PMID: 37631480 Free PMC article.
-
Poly(lactic acid)/Zinc/Alginate Complex Material: Preparation and Antimicrobial Properties.Antibiotics (Basel). 2021 Oct 30;10(11):1327. doi: 10.3390/antibiotics10111327. Antibiotics (Basel). 2021. PMID: 34827265 Free PMC article.
-
Synthesis and Modification of a Natural Polymer with the Participation of Metal Nanoparticles, Study of Their Composition and Properties.Polymers (Basel). 2024 Jan 18;16(2):264. doi: 10.3390/polym16020264. Polymers (Basel). 2024. PMID: 38257065 Free PMC article.
-
Antibiofilm Effects of Modifying Polyvinylidene Fluoride Membranes with Polyethylenimine, Poly(acrylic acid) and Graphene Oxide.Polymers (Basel). 2024 Dec 5;16(23):3418. doi: 10.3390/polym16233418. Polymers (Basel). 2024. PMID: 39684163 Free PMC article.
References
-
- Riegels N., Vairavamoorthy K., Herrick J., Kauppi L., Mcneely J.A., Mcglade J., Eboh E., Smith M., Acheampong E., Pengue W., et al. Options for Decoupling Economic Growth From Water Use and Water. [(accessed on 28 January 2021)];2015 Available online: http://idaea.csic.es/sites/default/files/Options_for_decoupling_economic....
-
- Nasir A., Masood F., Yasin T., Hameed A. Progress in polymeric nanocomposite membranes for wastewater treatment: Preparation, properties and applications. J. Ind. Eng. Chem. 2019;79:29–40. doi: 10.1016/j.jiec.2019.06.052. - DOI
-
- Shon H.K., Phuntsho S., Chaudhary D.S., Vigneswaran S., Cho J. Nanofiltration for water and wastewater treatment—A mini review. Drink. Water Eng. Sci. 2013;6:47–53. doi: 10.5194/dwes-6-47-2013. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

