Phage-Encoded Endolysins
- PMID: 33525684
- PMCID: PMC7912344
- DOI: 10.3390/antibiotics10020124
Phage-Encoded Endolysins
Abstract
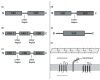
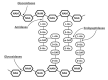
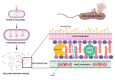
Due to the global emergence of antibiotic resistance, there has been an increase in research surrounding endolysins as an alternative therapeutic. Endolysins are phage-encoded enzymes, utilized by mature phage virions to hydrolyze the cell wall from within. There is significant evidence that proves the ability of endolysins to degrade the peptidoglycan externally without the assistance of phage. Thus, their incorporation in therapeutic strategies has opened new options for therapeutic application against bacterial infections in the human and veterinary sectors, as well as within the agricultural and biotechnology sectors. While endolysins show promising results within the laboratory, it is important to document their resistance, safety, and immunogenicity for in-vivo application. This review aims to provide new insights into the synergy between endolysins and antibiotics, as well as the formulation of endolysins. Thus, it provides crucial information for clinical trials involving endolysins.
Keywords: antibiotic resistance; bacteriophages; endolysin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

