MiR-144-3p-mediated dysregulation of EIF4G2 contributes to the development of hepatocellular carcinoma through the ERK pathway
- PMID: 33526055
- PMCID: PMC7852102
- DOI: 10.1186/s13046-021-01853-6
MiR-144-3p-mediated dysregulation of EIF4G2 contributes to the development of hepatocellular carcinoma through the ERK pathway
Abstract
Background: Hepatocellular carcinoma (HCC) is one of the most common cancers with high incidence and mortality. However, the underlying mechanisms of HCC still remain unclear. Eukaryotic translation initiation factors (eIFs) have a substantial effect on tumor development. In this study, we were aimed to investigate the role of eukaryotic translation initiation factor 4 gamma 2 (EIF4G2) in HCC.
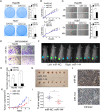
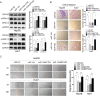
Methods: Western blot (WB) of 30 paired HCC tissues and tissue microarrays (TMAs) conducted by immunohistochemistry (IHC) in 89 paired HCC samples were performed to assess EIF4G2 expression. Clone formation, real-time cell analysis (RTCA), wound healing and transwell assays were adopted to evaluate the role of EIF4G2 on HCC cell proliferation, migration and invasion abilities. The function of EIF4G2 in HCC tumor growth was assessed in a xenograft nude mouse model in vivo. The regulation of EIF4G2 by miR-144-3p was performed by luciferase reporter assay and WB.
Results: The EIF4G2 protein was clearly upregulated in HCC tissues, and high EIF4G2 expression was closely related to HCC prognosis. EIF4G2 silencing could inhibit HCC cell growth and metastasis in vitro, and suppress tumorigenesis in vivo by repressing the ERK signaling pathway. The results of luciferase reporter assays, WB and IHC staining verified that EIF4G2 was negatively regulated by miR-144. And re-expression of EIF4G2 could partially reverse the inhibiting effect of miR-144 in HCC.
Conclusion: In summary, our study revealed the role of EIF4G2 in HCC development via the activation of the ERK pathway. We also found that EIF4G2 could be negatively regulated by the tumor suppressor miR-144. Our investigations indicated that EIF4G2 might be a promising therapeutic target in HCC.
Keywords: EIF4G2; ERK1/2; HCC; Prognosis; miR-144-3p.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

