Factors affecting serum phenobarbital concentration changes in pediatric patients receiving elixir and powder formulations
- PMID: 33526089
- PMCID: PMC7852295
- DOI: 10.1186/s40780-021-00190-2
Factors affecting serum phenobarbital concentration changes in pediatric patients receiving elixir and powder formulations
Abstract
Background: Phenobarbital (PB) is commonly used as elixir and powder formulations in pediatric care. Its dose adjustment is performed based on individual drug concentration monitoring. Few studies have comprehensively analyzed the variation factors for serum PB concentration. In this study, we retrospectively investigated the factors that influence serum PB concentration and assessed the impacts of dosage formulation and administration route.
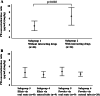
Methods: This retrospective cohort study covered clinical data from January 2007 to September 2019 at Mie University Hospital. The present study included 60 pediatric patients administered the elixir and powder of PB through oral route and enteral tube. Simple and multiple linear regression analyses were performed to identify the risk factors that affect the weight-corrected PB serum concentration/dose (C/D) ratio in pediatric patients. Six subgroups were also established according to the concomitant use of drugs that potentially inhibit PB metabolism, dosage formulation, and administration route to investigate the difference in the PB C/D ratio among the subgroups.
Results: A significant regression equation to predict the PB C/D ratio was found through simple and multiple linear regression analyses, with an adjusted coefficient of determination of 0.53 (p < 0.001). Further, the concomitant uses of valproic acid (VPA) or amiodarone, which were the only two drugs seen in this study as potential inhibitors of PB, was found to have the greatest effect on the PB C/D ratio (standardized partial regression coefficient (β) = 0.543, p < 0.001). Furthermore, a significant difference in the PB C/D ratio was found between the subgroups classified by the concomitant use of VPA or amiodarone (p = 0.002). However, there were no significant correlations between the PB C/D ratio, dosage formulation, and administration route.
Conclusions: The most influential factor on the PB C/D ratio was the concomitant use of VPA or amiodarone with PB. This result could provide an important perspective in pediatric drug therapy where elixir and powder formulations are administered via the oral route and enteral tube.
Keywords: Elixir; Pediatric care; Phenobarbital; Powder; Serum concentration.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures
References
-
- Hauptmann A. Luminal bei epilepsie. Munch Med Wochenschr. 1912;59:1907–1909.
-
- Tokiwa C, Nakajima M. The high blood concentration of phenobarbital after a change to elixir from powder. The Japanese Journal of Developmental Pharmacology and Therapeutics. 2018;31:50–53.
LinkOut - more resources
Full Text Sources
Other Literature Sources