The Cell Biology of LRRK2 in Parkinson's Disease
- PMID: 33526455
- PMCID: PMC8088268
- DOI: 10.1128/MCB.00660-20
The Cell Biology of LRRK2 in Parkinson's Disease
Abstract
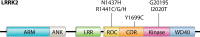
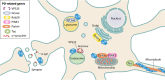
Point mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common cause of familial Parkinson's disease (PD) and are implicated in a significant proportion of apparently sporadic PD cases. Clinically, LRRK2-driven PD is indistinguishable from sporadic PD, making it an attractive genetic model for the much more common sporadic PD. In this review, we highlight recent advances in understanding LRRK2's subcellular functions using LRRK2-driven PD models, while also considering some of the limitations of these model systems. Recent developments of particular importance include new evidence of key LRRK2 functions in the endolysosomal system and LRRK2's regulation of and by Rab GTPases. Additionally, LRRK2's interaction with the cytoskeleton allowed elucidation of the LRRK2 structure and appears relevant to LRRK2 protein degradation and LRRK2 inhibitor therapies. We further discuss how LRRK2's interactions with other PD-driving genes, such as the VPS35, GBA1, and SNCA genes, may highlight cellular pathways more broadly disrupted in PD.
Keywords: LRRK2; Parkinson's disease; endolysosome; kinase; microtubule.
Copyright © 2021 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
