Palmitoylation Controls NMDA Receptor Function and Steroid Sensitivity
- PMID: 33526476
- PMCID: PMC8018758
- DOI: 10.1523/JNEUROSCI.2654-20.2021
Palmitoylation Controls NMDA Receptor Function and Steroid Sensitivity
Abstract
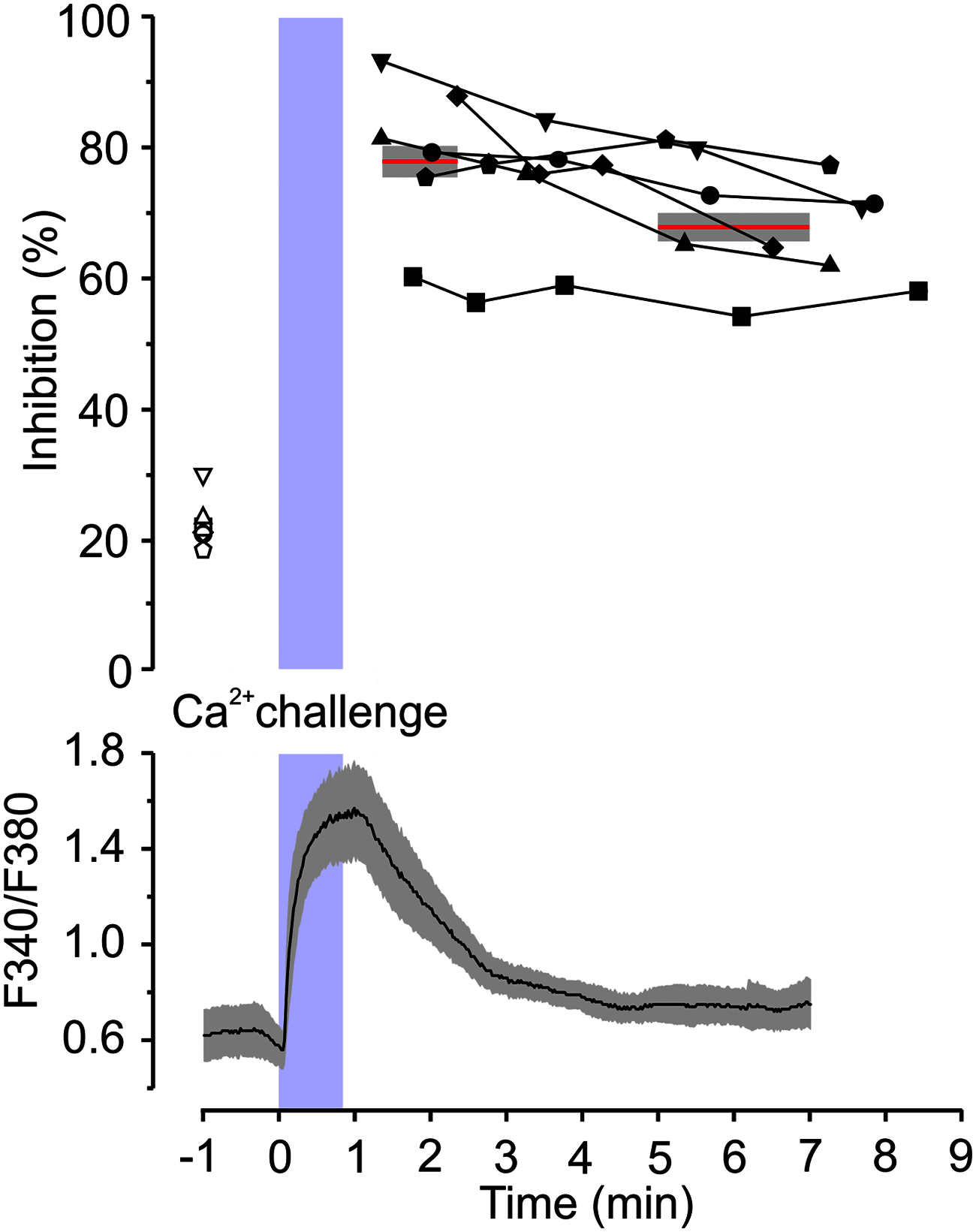
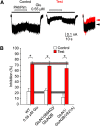
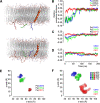
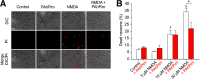
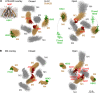
NMDARs are ligand-gated ion channels that cause an influx of Na+ and Ca2+ into postsynaptic neurons. The resulting intracellular Ca2+ transient triggers synaptic plasticity. When prolonged, it may induce excitotoxicity, but it may also activate negative feedback to control the activity of NMDARs. Here, we report that a transient rise in intracellular Ca2+ (Ca2+ challenge) increases the sensitivity of NMDARs but not AMPARs/kainate receptors to the endogenous inhibitory neurosteroid 20-oxo-5β-pregnan-3α-yl 3-sulfate and to its synthetic analogs, such as 20-oxo-5β-pregnan-3α-yl 3-hemipimelate (PAhPim). In cultured hippocampal neurons, 30 μm PAhPim had virtually no effect on NMDAR responses; however, following the Ca2+ challenge, it inhibited the responses by 62%; similarly, the Ca2+ challenge induced a 3.7-fold decrease in the steroid IC50 on recombinant GluN1/GluN2B receptors. The increase in the NMDAR sensitivity to PAhPim was dependent on three cysteines (C849, C854, and C871) located in the carboxy-terminal domain of the GluN2B subunit, previously identified to be palmitoylated (Hayashi et al., 2009). Our experiments suggested that the Ca2+ challenge induced receptor depalmitoylation, and single-channel analysis revealed that this was accompanied by a 55% reduction in the probability of channel opening. Results of in silico modeling indicate that receptor palmitoylation promotes anchoring of the GluN2B subunit carboxy-terminal domain to the plasma membrane and facilitates channel opening. Depalmitoylation-induced changes in the NMDAR pharmacology explain the neuroprotective effect of PAhPim on NMDA-induced excitotoxicity. We propose that palmitoylation-dependent changes in the NMDAR sensitivity to steroids serve as an acute endogenous mechanism that controls NMDAR activity.SIGNIFICANCE STATEMENT There is considerable interest in negative allosteric modulators of NMDARs that could compensate for receptor overactivation by glutamate or de novo gain-of-function mutations in neurodevelopmental disorders. By a combination of electrophysiological, pharmacological, and computational techniques we describe a novel feedback mechanism regulating NMDAR activity. We find that a transient rise in intracellular Ca2+ increases NMDAR sensitivity to inhibitory neurosteroids in a process dependent on GluN2B subunit depalmitoylation. These results improve our understanding of the molecular mechanisms of steroid action at the NMDAR and indeed of the basic properties of this important glutamate-gated ion channel and may aid in the development of therapeutics for treating neurologic and psychiatric diseases related to overactivation of NMDARs without affecting normal physiological functions.
Keywords: NMDAR; carboxy-terminal domain; molecular dynamics simulation; neurosteroid; palmitoylation; single-channel recording.
Copyright © 2021 the authors.
Figures



References
-
- Abraham MJ, Murtola T, Schulz R, Pall S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1:19–25. 10.1016/j.softx.2015.06.001 - DOI
-
- Adla SK, Slavikova B, Smidkova M, Tloustova E, Svoboda M, Vyklicky V, Krausova B, Hubalkova P, Nekardova M, Holubova K, Vales K, Budesinsky M, Vyklicky L, Chodounska H, Kudova E (2017) Physicochemical and biological properties of novel amide-based steroidal inhibitors of NMDA receptors. Steroids 117:52–61. 10.1016/j.steroids.2016.08.010 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
