Targeting Pan-ETS Factors Inhibits Melanoma Progression
- PMID: 33526511
- PMCID: PMC8137525
- DOI: 10.1158/0008-5472.CAN-19-1668
Targeting Pan-ETS Factors Inhibits Melanoma Progression
Abstract
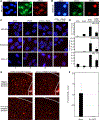
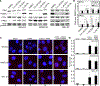
The failure of once promising target-specific therapeutic strategies often arises from redundancies in gene expression pathways. Even with new melanoma treatments, many patients are not responsive or develop resistance, leading to disease progression in terms of growth and metastasis. We previously discovered that the transcription factors ETS1 and PAX3 drive melanoma growth and metastasis by promoting the expression of the MET receptor. Here, we find that there are multiple ETS family members expressed in melanoma and that these factors have redundant functions. The small molecule YK-4-279, initially developed to target the ETS gene-containing translocation product EWS-FLI1, significantly inhibited cellular growth, invasion, and ETS factor function in melanoma cell lines and a clinically relevant transgenic mouse model, BrafCA;Tyr-CreERT2;Ptenf/f. One of the antitumor effects of YK-4-279 in melanoma is achieved via interference of multiple ETS family members with PAX3 and the expression of the PAX3-ETS downstream gene MET. Expression of exogenous MET provided partial rescue of the effects of YK-4-279, further supporting that MET loss is a significant contributor to the antitumor effects of the drug. This is the first study identifying multiple overlapping functions of the ETS family promoting melanoma. In addition, targeting all factors, rather than individual members, demonstrated impactful deleterious consequences in melanoma progression. Given that multiple ETS factors are known to have oncogenic functions in other malignancies, these findings have a high therapeutic impact. SIGNIFICANCE: These findings identify YK-4-279 as a promising therapeutic agent against melanoma by targeting multiple ETS family members and blocking their ability to act as transcription factors.
©2021 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest statement: The authors have declared that no conflict of interest exists.
Figures





References
-
- Keehn CA, Smoller BR, and Morgan MB. Expression of the ets-1 proto-oncogene in melanocytic lesions. Mod Pathol. 2003;16(8):772–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

