Chromatophores efficiently promote light-driven ATP synthesis and DNA transcription inside hybrid multicompartment artificial cells
- PMID: 33526592
- PMCID: PMC7896284
- DOI: 10.1073/pnas.2012170118
Chromatophores efficiently promote light-driven ATP synthesis and DNA transcription inside hybrid multicompartment artificial cells
Abstract
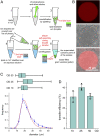
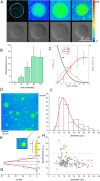
The construction of energetically autonomous artificial protocells is one of the most ambitious goals in bottom-up synthetic biology. Here, we show an efficient manner to build adenosine 5'-triphosphate (ATP) synthesizing hybrid multicompartment protocells. Bacterial chromatophores from Rhodobacter sphaeroides accomplish the photophosphorylation of adenosine 5'-diphosphate (ADP) to ATP, functioning as nanosized photosynthetic organellae when encapsulated inside artificial giant phospholipid vesicles (ATP production rate up to ∼100 ATP∙s-1 per ATP synthase). The chromatophore morphology and the orientation of the photophosphorylation proteins were characterized by cryo-electron microscopy (cryo-EM) and time-resolved spectroscopy. The freshly synthesized ATP has been employed for sustaining the transcription of a DNA gene, following the RNA biosynthesis inside individual vesicles by confocal microscopy. The hybrid multicompartment approach here proposed is very promising for the construction of full-fledged artificial protocells because it relies on easy-to-obtain and ready-to-use chromatophores, paving the way for artificial simplified-autotroph protocells (ASAPs).
Keywords: artificial photosynthesis; artificial protocells; bacterial chromatophores; light transduction; synthetic biology.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Luisi P. L., Toward the engineering of minimal living cells. Anat. Rec. 268, 208–214 (2002). - PubMed
-
- Schwille P., et al., MaxSynBio: Avenues towards creating cells from the bottom up. Angew. Chem. Int. Ed. Engl. 57, 13382–13392 (2018). - PubMed
-
- Blain J. C., Szostak J. W., Progress toward synthetic cells. Annu. Rev. Biochem. 83, 615–640 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

