STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment
- PMID: 33526609
- PMCID: PMC7852948
- DOI: 10.1136/jitc-2020-001906
STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment
Abstract
Background: The degree of immune infiltration in tumors, especially CD8+ T cells, greatly impacts patient disease course and response to interventional immunotherapy. Enhancement of tumor infiltrating lymphocyte (TIL) is a critical element of efficacious therapy and one that may be achieved via administration of agents that promote tumor vascular normalization (VN) and/or induce the development of tertiary lymphoid structures (TLS) within the tumor microenvironment (TME).
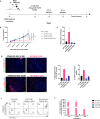
Methods: Low-dose stimulator of interferon genes (STING) agonist ADU S-100 (5 µg/mouse) was delivered intratumorally to established subcutaneous B16.F10 melanomas on days 10, 14 and 17 post-tumor inoculation. Treated and control tumors were isolated at various time points to assess transcriptional changes associated with VN and TLS formation via quantitative PCR (qPCR), with corollary immune cell composition changes in isolated tissues determined using flow cytometry and immunofluorescence microscopy. In vitro assays were performed on CD11c+ BMDCs treated with 2.5 µg/mL ADU S-100 or CD11c+ DCs isolated from tumor digests and associated transcriptional changes analyzed via qPCR or profiled using DNA microarrays. For T cell repertoireβ-CDR3 analyses, T cell CDR3 was sequenced from gDNA isolated from splenocytes and enzymatically digested tumors.
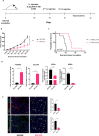
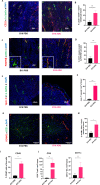
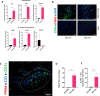
Results: We report that activation of STING within the TME leads to slowed melanoma growth in association with increased production of antiangiogenic factors including Tnfsf15 (Vegi) and Cxcl10, and TLS-inducing factors including Ccl19, Ccl21, Lta, Ltb and Light. Therapeutic responses resulting from intratumoral STING activation were characterized by improved VN, enhanced tumor infiltration by CD8+ T cells and CD11c+ DCs and local TLS neogenesis, all of which were dependent on host expression of STING. Consistent with a central role for DC in TLS formation, ADU S-100-activated mCD11c+ DCs also exhibited upregulated expression of TLS promoting factors including lymphotoxin-α (LTA), interleukin (IL)-36, inflammatory chemokines and type I interferons in vitro and in vivo. TLS formation in ADU S-100-treated mice was associated with the development of a highly oligoclonal TIL repertoire enriched in expanded T cell clonotypes unique to the TME and not detected in the periphery.
Conclusions: Our data support the premise that i.t. delivery of low-dose STING agonist promotes VN and a proinflammatory TME supportive of TLS formation, enrichment in the TIL repertoire and tumor growth control.
Keywords: CD8-Positive T-Lymphocytes; dendritic cells; immunohistochemistry; immunotherapy; melanoma.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Society AC ACS facts and figures 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
