Area-specific thalamocortical synchronization underlies the transition from motor planning to execution
- PMID: 33526664
- PMCID: PMC8017695
- DOI: 10.1073/pnas.2012658118
Area-specific thalamocortical synchronization underlies the transition from motor planning to execution
Abstract
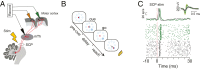
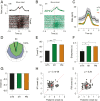
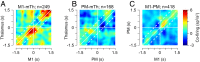
We studied correlated firing between motor thalamic and cortical cells in monkeys performing a delayed-response reaching task. Simultaneous recording of thalamocortical activity revealed that around movement onset, thalamic cells were positively correlated with cell activity in the primary motor cortex but negatively correlated with the activity of the premotor cortex. The differences in the correlation contrasted with the average neural responses, which were similar in all three areas. Neuronal correlations reveal functional cooperation and opposition between the motor thalamus and distinct motor cortical areas with specific roles in planning vs. performing movements. Thus, by enhancing and suppressing motor and premotor firing, the motor thalamus can facilitate the transition from a motor plan to execution.
Keywords: cerebellum; motor cortex; motor thalamus; movement initiation; nonhuman primates.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Strick P., Sterling P., Synaptic termination of afferents from the ventrolateral nucleus of the thalamus in the cat motor cortex. A light and electron microscopy study. J. Comp. Neurol. 153, 77–106 (1974). - PubMed
-
- Strick P. L., Light microscopic analysis of the cortical projection of the thalamic ventrolateral nucleus in the cat. Brain Res. 55, 1–24 (1973). - PubMed
-
- Nashef A., Cohen O., Harel R., Israel Z., Prut Y., Reversible block of cerebellar outflow reveals cortical circuitry for motor coordination. Cell Rep. 27, 2608–2619.e4 (2019). - PubMed
-
- Dacre J., et al. , Cerebellar-recipient motor thalamus drives behavioral context-specific movement initiation (2019). dx.doi.org/10.2139/ssrn.3470398. Accessed 14 January 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

