Identifying hydrophobic protein patches to inform protein interaction interfaces
- PMID: 33526682
- PMCID: PMC8018078
- DOI: 10.1073/pnas.2018234118
Identifying hydrophobic protein patches to inform protein interaction interfaces
Abstract
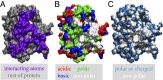
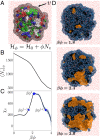
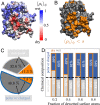
Interactions between proteins lie at the heart of numerous biological processes and are essential for the proper functioning of the cell. Although the importance of hydrophobic residues in driving protein interactions is universally accepted, a characterization of protein hydrophobicity, which informs its interactions, has remained elusive. The challenge lies in capturing the collective response of the protein hydration waters to the nanoscale chemical and topographical protein patterns, which determine protein hydrophobicity. To address this challenge, here, we employ specialized molecular simulations wherein water molecules are systematically displaced from the protein hydration shell; by identifying protein regions that relinquish their waters more readily than others, we are then able to uncover the most hydrophobic protein patches. Surprisingly, such patches contain a large fraction of polar/charged atoms and have chemical compositions that are similar to the more hydrophilic protein patches. Importantly, we also find a striking correspondence between the most hydrophobic protein patches and regions that mediate protein interactions. Our work thus establishes a computational framework for characterizing the emergent hydrophobicity of amphiphilic solutes, such as proteins, which display nanoscale heterogeneity, and for uncovering their interaction interfaces.
Keywords: PPI; dewetting; hydration; hydrophobicity; proteins.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Trevino S. R., Scholtz J. M., Pace C. N., Measuring and increasing protein solubility. J. Pharmacol. Sci. 97, 4155–4166 (2008). - PubMed
-
- Scott D. E., Bayly A. R., Abell C., Skidmore J., Small molecules, big targets: Drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 15, 533–550 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

