Importin α phosphorylation promotes TPX2 activation by GM130 to control astral microtubules and spindle orientation
- PMID: 33526712
- PMCID: PMC7904092
- DOI: 10.1242/jcs.258356
Importin α phosphorylation promotes TPX2 activation by GM130 to control astral microtubules and spindle orientation
Abstract
Spindle orientation is important in multiple developmental processes as it determines cell fate and function. The orientation of the spindle depends on the assembly of a proper astral microtubule network. Here, we report that the spindle assembly factor TPX2 regulates astral microtubules. TPX2 in the spindle pole area is activated by GM130 (GOLGA2) on Golgi membranes to promote astral microtubule growth. GM130 relieves TPX2 inhibition by competing for importin α1 (KPNA2) binding. Mitotic phosphorylation of importin α at serine 62 (S62) by CDK1 switches its substrate preference from TPX2 to GM130, thereby enabling competition-based activation. Importin α S62A mutation impedes local TPX2 activation and compromises astral microtubule formation, ultimately resulting in misoriented spindles. Blocking the GM130-importin α-TPX2 pathway impairs astral microtubule growth. Our results reveal a novel role for TPX2 in the organization of astral microtubules. Furthermore, we show that the substrate preference of the important mitotic modulator importin α is regulated by CDK1-mediated phosphorylation.
Keywords: Astral microtubules; CDK1; GM130; Golgi; Importin α; Mitosis; Phosphorylation; Spindle orientation; TPX2.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


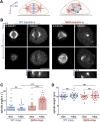
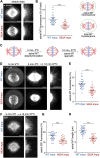



Similar articles
-
Phosphorylation of importin-α1 by CDK1-cyclin B1 controls mitotic spindle assembly.J Cell Sci. 2019 Sep 23;132(18):jcs232314. doi: 10.1242/jcs.232314. J Cell Sci. 2019. PMID: 31434716 Free PMC article.
-
GM130 Regulates Golgi-Derived Spindle Assembly by Activating TPX2 and Capturing Microtubules.Cell. 2015 Jul 16;162(2):287-299. doi: 10.1016/j.cell.2015.06.014. Epub 2015 Jul 9. Cell. 2015. PMID: 26165940 Free PMC article.
-
Destabilization of Long Astral Microtubules via Cdk1-Dependent Removal of GTSE1 from Their Plus Ends Facilitates Prometaphase Spindle Orientation.Curr Biol. 2021 Feb 22;31(4):766-781.e8. doi: 10.1016/j.cub.2020.11.040. Epub 2020 Dec 16. Curr Biol. 2021. PMID: 33333009
-
TPX2: of spindle assembly, DNA damage response, and cancer.Cell Mol Life Sci. 2014 Aug;71(16):3027-47. doi: 10.1007/s00018-014-1582-7. Epub 2014 Feb 21. Cell Mol Life Sci. 2014. PMID: 24556998 Free PMC article. Review.
-
Contribution of AurkA/TPX2 Overexpression to Chromosomal Imbalances and Cancer.Cells. 2024 Aug 22;13(16):1397. doi: 10.3390/cells13161397. Cells. 2024. PMID: 39195284 Free PMC article. Review.
Cited by
-
Oxidative stress mediates nucleocytoplasmic shuttling of KPNA2 via AKT1-CDK1 axis-regulated S62 phosphorylation.FASEB Bioadv. 2024 Jul 5;6(8):276-288. doi: 10.1096/fba.2024-00078. eCollection 2024 Aug. FASEB Bioadv. 2024. PMID: 39114447 Free PMC article.
-
Structural Organization and Function of the Golgi Ribbon During Cell Division.Front Cell Dev Biol. 2022 Jun 24;10:925228. doi: 10.3389/fcell.2022.925228. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35813197 Free PMC article.
-
The Role of GM130 in Nervous System Diseases.Front Neurol. 2021 Oct 28;12:743787. doi: 10.3389/fneur.2021.743787. eCollection 2021. Front Neurol. 2021. PMID: 34777211 Free PMC article. Review.
-
Palmitoylated importin α regulates mitotic spindle orientation through interaction with NuMA.EMBO Rep. 2025 Jul;26(13):3280-3304. doi: 10.1038/s44319-025-00484-8. Epub 2025 May 27. EMBO Rep. 2025. PMID: 40425783 Free PMC article.
-
Annexin A1 is a polarity cue that directs mitotic spindle orientation during mammalian epithelial morphogenesis.Nat Commun. 2023 Jan 11;14(1):151. doi: 10.1038/s41467-023-35881-x. Nat Commun. 2023. PMID: 36631478 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

