Calcium channel ITPR2 and mitochondria-ER contacts promote cellular senescence and aging
- PMID: 33526781
- PMCID: PMC7851384
- DOI: 10.1038/s41467-021-20993-z
Calcium channel ITPR2 and mitochondria-ER contacts promote cellular senescence and aging
Abstract
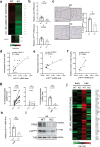
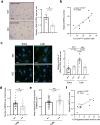
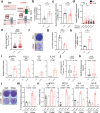
Cellular senescence is induced by stresses and results in a stable proliferation arrest accompanied by a pro-inflammatory secretome. Senescent cells accumulate during aging, promoting various age-related pathologies and limiting lifespan. The endoplasmic reticulum (ER) inositol 1,4,5-trisphosphate receptor, type 2 (ITPR2) calcium-release channel and calcium fluxes from the ER to the mitochondria are drivers of senescence in human cells. Here we show that Itpr2 knockout (KO) mice display improved aging such as increased lifespan, a better response to metabolic stress, less immunosenescence, as well as less liver steatosis and fibrosis. Cellular senescence, which is known to promote these alterations, is decreased in Itpr2 KO mice and Itpr2 KO embryo-derived cells. Interestingly, ablation of ITPR2 in vivo and in vitro decreases the number of contacts between the mitochondria and the ER and their forced contacts induce premature senescence. These findings shed light on the role of contacts and facilitated exchanges between the ER and the mitochondria through ITPR2 in regulating senescence and aging.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Getting old without type 2 IP3 receptors.Cell Calcium. 2021 Sep;98:102437. doi: 10.1016/j.ceca.2021.102437. Epub 2021 Jun 24. Cell Calcium. 2021. PMID: 34252746 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

