A glucose meter interface for point-of-care gene circuit-based diagnostics
- PMID: 33526784
- PMCID: PMC7851131
- DOI: 10.1038/s41467-020-20639-6
A glucose meter interface for point-of-care gene circuit-based diagnostics
Abstract
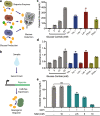
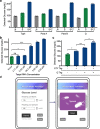
Recent advances in cell-free synthetic biology have given rise to gene circuit-based sensors with the potential to provide decentralized and low-cost molecular diagnostics. However, it remains a challenge to deliver this sensing capacity into the hands of users in a practical manner. Here, we leverage the glucose meter, one of the most widely available point-of-care sensing devices, to serve as a universal reader for these decentralized diagnostics. We describe a molecular translator that can convert the activation of conventional gene circuit-based sensors into a glucose output that can be read by off-the-shelf glucose meters. We show the development of new glucogenic reporter systems, multiplexed reporter outputs and detection of nucleic acid targets down to the low attomolar range. Using this glucose-meter interface, we demonstrate the detection of a small-molecule analyte; sample-to-result diagnostics for typhoid, paratyphoid A/B; and show the potential for pandemic response with nucleic acid sensors for SARS-CoV-2.
Conflict of interest statement
The Governing Council of the University of Toronto has filed a patent application covering the invention of the gene circuit-glucose meter interface with inventors Evan Amalfitano, Margot Karlikow and Keith Pardee (International application number PCT/CA2018/051646, provisional status). Arizona State University has filed a patent application covering the invention of the SARS-CoV-2 sensors with Masoud Norouzi, Margot Karlikow, Alexander Green and Keith Pardee (US patent number 63/038,609, provisional status). The remaining authors declare no competing interests.
Figures

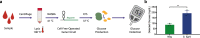

References
-
- Wu M-R, Jusiak B, Lu TK. Engineering advanced cancer therapies with synthetic biology. Nat. Rev. Cancer. 2019;19:187–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

