BTK gatekeeper residue variation combined with cysteine 481 substitution causes super-resistance to irreversible inhibitors acalabrutinib, ibrutinib and zanubrutinib
- PMID: 33526860
- PMCID: PMC8102192
- DOI: 10.1038/s41375-021-01123-6
BTK gatekeeper residue variation combined with cysteine 481 substitution causes super-resistance to irreversible inhibitors acalabrutinib, ibrutinib and zanubrutinib
Abstract
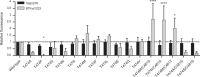
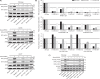
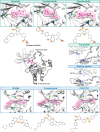
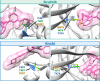
Irreversible inhibitors of Bruton tyrosine kinase (BTK), pioneered by ibrutinib, have become breakthrough drugs in the treatment of leukemias and lymphomas. Resistance variants (mutations) occur, but in contrast to those identified for many other tyrosine kinase inhibitors, they affect less frequently the "gatekeeper" residue in the catalytic domain. In this study we carried out variation scanning by creating 11 substitutions at the gatekeeper amino acid, threonine 474 (T474). These variants were subsequently combined with replacement of the cysteine 481 residue to which irreversible inhibitors, such as ibrutinib, acalabrutinib and zanubrutinib, bind. We found that certain double mutants, such as threonine 474 to isoleucine (T474I) or methionine (T474M) combined with catalytically active cysteine 481 to serine (C481S), are insensitive to ≥16-fold the pharmacological serum concentration, and therefore defined as super-resistant to irreversible inhibitors. Conversely, reversible inhibitors showed a variable pattern, from resistance to no resistance, collectively demonstrating the structural constraints for different classes of inhibitors, which may affect their clinical application.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


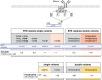
References
-
- Smith CIE, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. BioEssays. 2001;23:436–46.. - PubMed
-
- Rawlings DJ. Bruton’s tyrosine kinase controls a sustained calcium signal essential for B lineage development and function. Clin Immunol. 1999;91:243–53. - PubMed
-
- Vetrie D, Vořechovský I, Sideras P, Holland J, Davies A, Flinter F, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33.. - PubMed
-
- Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

