Novel variants in the stem cell niche factor WNT2B define the disease phenotype as a congenital enteropathy with ocular dysgenesis
- PMID: 33526876
- PMCID: PMC8187348
- DOI: 10.1038/s41431-021-00812-1
Novel variants in the stem cell niche factor WNT2B define the disease phenotype as a congenital enteropathy with ocular dysgenesis
Abstract
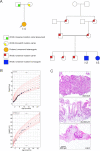
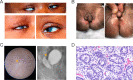
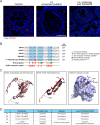
WNT2B is a member of the Wnt family, a group of signal transduction proteins involved in embryologic development and stem cell renewal and maintenance. We recently reported homozygous nonsense variants in WNT2B in three individuals with severe, neonatal-onset diarrhea, and intestinal failure. Here we present a fourth case, from a separate family, with neonatal diarrhea associated with novel compound heterozygous WNT2B variants. One of the two variants was a frameshift variant (c.423del [p.Phe141fs]), while the other was a missense change (c.722 G > A [p.G241D]) that we predict through homology modeling to be deleterious, disrupting post-translational acylation. This patient presented as a neonate with severe diet-induced (osmotic) diarrhea and growth failure resulting in dependence on parenteral nutrition. Her gastrointestinal histology revealed abnormal cellular architecture particularly in the stomach and colon, including oxyntic atrophy, abnormal distribution of enteroendocrine cells, and a paucity of colonic crypt glands. In addition to her gastrointestinal findings, she had bilateral corneal clouding and atypical genital development later identified as a testicular 46,XX difference/disorder of sexual development. Upon review of the previously reported cases, two others also had anterior segment ocular anomalies though none had atypical genital development. This growing case series suggests that variants in WNT2B are associated with an oculo-intestinal (and possibly gonadal) syndrome, due to the protein's putative involvement in multiple developmental and stem cell maintenance pathways.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

