SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada
- PMID: 33527398
- PMCID: PMC8013879
- DOI: 10.1111/trf.16296
SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada
Abstract
Background: Case detection underestimates the burden of the COVID-19 pandemic. Following the first COVID-19 wave, we estimated the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among blood donors across Canada.
Study design and methods: This serial cross-sectional study was conducted between May 9 and July 21, 2020 from blood donors donating at all Canadian Blood Services locations. We used the Abbott Architect assay to detect SARS-CoV-2 IgG antibodies from retention plasma. Seroprevalence was standardized to population-level demographics and assay characteristics were adjusted using the Rogan-Gladen equation. Results were stratified by region, age, ethnicity, ABO groups, and quantiles of material and social deprivation indices. Temporal trends were evaluated at 2-week intervals. Univariate and multivariate logistic regression compared SARS-CoV-2 reactive to non-reactive donors by sociodemographic variables.
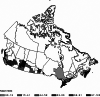
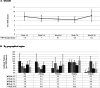
Results: Overall 552/74642 donors, had detectable antibodies, adjusted seroprevalence was 7.0/1000 donors (95% CI; 6.3, 7.6). Prevalence was differential by geography, Ontario had the highest rate, 8.8/1000 donors (7.8, 9.8), compared to the Atlantic region 4.5/1000 donors (2.6, 6.4); adjusted odds ratio (aOR) 2.2 (1.5, 3.3). Donors that self-identified as an ethnic minority were more likely than white donors to be sero-reactive aOR 1.5 (1.2, 1.9). No temporal trends were observed.
Discussion: Worldwide, blood services have leveraged their operational capacity to inform public health. While >99% of Canadians did not show humoral evidence of past infection, we found regional variability and disparities by ethnicity. Seroprevalence studies will continue to play a pivotal role in evaluating public health policies by identifying trends and monitor disparities.
Keywords: COVID-19; Canada; SARS-CoV-2 seroprevalence; blood donors.
© 2021 AABB.
Conflict of interest statement
SJD has acted as a content expert for respiratory viruses for Johnson & Johnson (Janssen). The remaining authors have no conflicts of interest to disclose.
Figures
References
-
- Detsky AS, Bogoch II. COVID‐19 in Canada: Experience and response. JAMA. 2020;324(8):743–4. - PubMed
-
- Coronavirus Disease (COVID‐19) : Outbreak update. Available from: https://www.canada.ca/en/public‐health/services/diseases/2019‐novel‐coro...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

