SARS-CoV-2-specific humoral and cellular immunity in two renal transplants and two hemodialysis patients treated with convalescent plasma
- PMID: 33527424
- PMCID: PMC8014298
- DOI: 10.1002/jmv.26840
SARS-CoV-2-specific humoral and cellular immunity in two renal transplants and two hemodialysis patients treated with convalescent plasma
Abstract
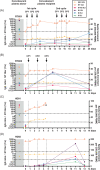
When patients with chronic kidney disease are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) they can face two specific problems: virus-specific immune responses may be impaired and remdesivir, an antiviral drug described to shorten recovery, is contraindicated. Antiviral treatment with convalescent plasma (CP) could be an alternative treatment option. In this case report, we present two kidney transplant recipients and two hemodialysis patients who were infected with SARS-CoV-2 and received CP. Antibodies against the receptor-binding domain in the S1 subunit of the SARS-CoV-2 spike protein were determined sequentially by immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) and neutralization assay and specific cellular responses by interferon-gamma ELISpot. Before treatment, in both kidney transplant recipients and one hemodialysis patient antibodies were undetectable by ELISA (ratio < 1.1), corresponding to low neutralizing antibody titers (≤1:40). ELISpot responses in the four patients were either weak or absent. After CP treatment, we observed an increase of SARS-CoV-2-specific antibodies (IgG ratio and neutralization titer) and of specific cellular responses. After intermittent clinical improvement, one kidney transplant recipient again developed typical symptoms on Day 12 after treatment and received a second cycle of CP treatment. Altogether, three patients clinically improved and could be discharged from the hospital. However, one 83-year-old multimorbid patient deceased. Our data suggest that the success of CP therapy may only be temporary in patients with chronic kidney disease; which requires close monitoring of viral load and antiviral immunity and possibly an adaptation of the treatment regimen.
Keywords: COVID-19; ELISpot; cellular immunity; convalescent plasma; hemodialysis; kidney transplantation.
© 2021 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures

Similar articles
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
SARS-CoV-2 Infection of Rhesus Macaques Treated Early with Human COVID-19 Convalescent Plasma.Microbiol Spectr. 2021 Dec 22;9(3):e0139721. doi: 10.1128/Spectrum.01397-21. Epub 2021 Nov 24. Microbiol Spectr. 2021. PMID: 34817208 Free PMC article.
-
Functional convalescent plasma antibodies and pre-infusion titers shape the early severe COVID-19 immune response.Nat Commun. 2021 Nov 25;12(1):6853. doi: 10.1038/s41467-021-27201-y. Nat Commun. 2021. PMID: 34824251 Free PMC article.
-
Convalescent plasma - Is it useful for treating SARS Co-V2 infection?Indian J Med Microbiol. 2020 Jul-Dec;38(3 & 4):252-260. doi: 10.4103/ijmm.IJMM_20_358. Indian J Med Microbiol. 2020. PMID: 33154232 Free PMC article. Review.
-
Antibody Responses in COVID-19: A Review.Front Immunol. 2021 Apr 15;12:633184. doi: 10.3389/fimmu.2021.633184. eCollection 2021. Front Immunol. 2021. PMID: 33936045 Free PMC article. Review.
Cited by
-
Convalescent plasma therapy and long-term SARS-COV-2 antiviral immune response in a prospective cohort of patients with COVID-19.Curr Res Microb Sci. 2025 Jul 9;9:100440. doi: 10.1016/j.crmicr.2025.100440. eCollection 2025. Curr Res Microb Sci. 2025. PMID: 40740192 Free PMC article.
-
Patient-blood management for COVID19 convalescent plasma therapy: relevance of affinity and donor-recipient differences in concentration of neutralizing antibodies.Clin Microbiol Infect. 2021 Jul;27(7):987-992. doi: 10.1016/j.cmi.2021.04.003. Epub 2021 Apr 17. Clin Microbiol Infect. 2021. PMID: 33878505 Free PMC article. Review.
-
Use of convalescent plasma in COVID-19 patients with immunosuppression.Transfusion. 2021 Aug;61(8):2503-2511. doi: 10.1111/trf.16525. Epub 2021 Jun 1. Transfusion. 2021. PMID: 34036587 Free PMC article. Review.
-
Lessons learned from the use of convalescent plasma for the treatment of COVID-19 and specific considerations for immunocompromised patients.Transfus Apher Sci. 2022 Jun;61(3):103355. doi: 10.1016/j.transci.2022.103355. Epub 2022 Jan 13. Transfus Apher Sci. 2022. PMID: 35063360 Free PMC article.
-
Long-Term SARS-CoV-2 Specific Immunity Is Affected by the Severity of Initial COVID-19 and Patient Age.J Clin Med. 2021 Oct 8;10(19):4606. doi: 10.3390/jcm10194606. J Clin Med. 2021. PMID: 34640623 Free PMC article.
References
-
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—Preliminary report. N Engl J Med. 2020;383:1813‐1826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

