Cell-Inspired Biomaterials for Modulating Inflammation
- PMID: 33528306
- PMCID: PMC9063153
- DOI: 10.1089/ten.TEB.2020.0276
Cell-Inspired Biomaterials for Modulating Inflammation
Abstract
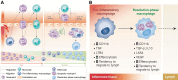
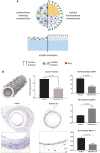
Inflammation is a crucial part of wound healing and pathogen clearance. However, it can also play a role in exacerbating chronic diseases and cancer progression when not regulated properly. A subset of current innate immune engineering research is focused on how molecules such as lipids, proteins, and nucleic acids native to a healthy inflammatory response can be harnessed in the context of biomaterial design to promote healing, decrease disease severity, and prolong survival. The engineered biomaterials in this review inhibit inflammation by releasing anti-inflammatory cytokines, sequestering proinflammatory cytokines, and promoting phenotype switching of macrophages in chronic inflammatory disease models. Conversely, other biomaterials discussed here promote inflammation by mimicking pathogen invasion to inhibit tumor growth in cancer models. The form that these biomaterials take spans a spectrum from nanoparticles to large-scale hydrogels to surface coatings on medical devices. Cell-inspired molecules have been incorporated in a variety of creative ways, including loaded into or onto the surface of biomaterials or used as the biomaterials themselves. Impact statement Chronic inflammatory diseases and cancers are widespread health care concerns. Treatment plans for these diseases can be complicated and the outcomes are often mixed due to off-target effects. Current research efforts in immune engineering and biomaterials are focused on utilizing the body's native immune response to return to homeostasis as a therapeutic approach. This review collects many of the most current findings in the field as a resource for future research.
Keywords: biomaterial; cancer; cell-inspired; inflammation; macrophage; nanoparticle.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Ovais, M., Guo, M., and Chen, C.. Tailoring nanomaterials for targeting tumor-associated macrophages. Adv Mater 31, 1808303, 2019. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

