WeChat as a Platform for Baduanjin Intervention in Patients With Stable Chronic Obstructive Pulmonary Disease in China: Retrospective Randomized Controlled Trial
- PMID: 33528369
- PMCID: PMC7886617
- DOI: 10.2196/23548
WeChat as a Platform for Baduanjin Intervention in Patients With Stable Chronic Obstructive Pulmonary Disease in China: Retrospective Randomized Controlled Trial
Abstract
Background: Pulmonary rehabilitation is a crucial part of the nonpharmacological treatment of stable chronic obstructive pulmonary disease (COPD), but management remains problematic. WeChat could serve as a useful tool in patient management. Baduanjin is a popular exercise in China that is usually applied in pulmonary rehabilitation, which has been confirmed to be effective in improving lung function and life quality.
Objective: This study aimed to explore the efficiency of WeChat in the management of Baduanjin exercise in COPD patients.
Methods: A total of 200 patients from the respiratory department of Putuo Hospital participated in the Baduanjin rehabilitation project from September 2018 to October 2019, and were randomly assigned to the WeChat and control groups and followed up using the WeChat platform or telephone for 12 weeks. The frequency of Baduanjin exercise, lung function (percentage of forced expiratory volume in 1 second predicted, FEV1% predicted), and COPD assessment test (CAT) scores were collected and compared between the two groups. The number of message exchanges and a satisfaction survey on the WeChat platform were used to assess the feasibility of WeChat management outside the hospital.
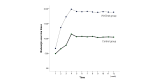
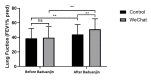
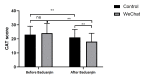
Results: The Baduanjin exercise frequency significantly differed between the control group and WeChat group (F=33.82, P<.001) and across various time points (F=214.87, P<.001). After the follow-up on WeChat, there were fewer patients not performing Baduanjin exercise. The FEV1% predicted value significantly differed before and after Baduanjin exercise in the control group (Z=-3.686, P<.001) and the WeChat group (Z=-6.985, P<.001). A significant difference in the FEV1% predicted value was observed after Baduanjin exercise between the two groups (Z=-3.679, P<.001). The CAT score significantly differed before and after Baduanjin exercise in the control group (Z=-4.937, P<.001) and the WeChat group (Z=-5.246, P<.001). A significant difference in the CAT score was observed after Baduanjin exercise between the two groups (Z=-5.246, P<.001). The number of completed Baduanjin exercises, lung function, and CAT scores in active patients were higher than those in nonactive patients. All satisfaction survey items were scored with more than 4 points. Among the items, the highest score (mean 4.54, SD 0.77) was for continued WeChat management, followed by the effective management of Baduanjin exercise (mean 4.46, SD 0.87). The patients in the WeChat group showed much higher enthusiasm for and compliance with Baduanjin exercise, resulting in better life quality and lung function. The patients were very satisfied with the WeChat management because of the obvious curative effect and home feeling.
Conclusions: The WeChat platform provided a feasible, effective, and sustainable management plan for Baduanjin rehabilitation.
Trial registration: Chinese Clinical Trial Registry ChiCTR1900028248; http://www.chictr.org.cn/showprojen.aspx?proj=46995.
Keywords: Baduanjin rehabilitation; WeChat management; chronic obstructive pulmonary disease.
©Junjie Bi, Wei Yang, Ping Hao, Yongmei Zhao, Dan Wei, Yipeng Sun, Yuhua Lin, Meng Sun, Xuan Chen, Xuming Luo, Shanqun Li, Wei Zhang, Xiongbiao Wang. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 02.02.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Dobler CC, Morrow AS, Beuschel B, Farah MH, Majzoub AM, Wilson ME, Hasan B, Seisa MO, Daraz L, Prokop LJ, Murad MH, Wang Z. Pharmacologic Therapies in Patients With Exacerbation of Chronic Obstructive Pulmonary Disease. Ann Intern Med. 2020 Feb 25;172(6):413–422. doi: 10.7326/M19-3007. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

