Association Between Dispatch of Mobile Stroke Units and Functional Outcomes Among Patients With Acute Ischemic Stroke in Berlin
- PMID: 33528537
- PMCID: PMC7856548
- DOI: 10.1001/jama.2020.26345
Association Between Dispatch of Mobile Stroke Units and Functional Outcomes Among Patients With Acute Ischemic Stroke in Berlin
Erratum in
-
Group Name Omitted.JAMA. 2021 Apr 6;325(13):1335. doi: 10.1001/jama.2021.2565. JAMA. 2021. PMID: 33821920 Free PMC article. No abstract available.
Abstract
Importance: Effects of thrombolysis in acute ischemic stroke are time-dependent. Ambulances that can administer thrombolysis (mobile stroke units [MSUs]) before arriving at the hospital have been shown to reduce time to treatment.
Objective: To determine whether dispatch of MSUs is associated with better clinical outcomes for patients with acute ischemic stroke.
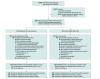
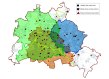
Design, setting, and participants: This prospective, nonrandomized, controlled intervention study was conducted in Berlin, Germany, from February 1, 2017, to October 30, 2019. If an emergency call prompted suspicion of stroke, both a conventional ambulance and an MSU, when available, were dispatched. Functional outcomes of patients with final diagnosis of acute cerebral ischemia who were eligible for thrombolysis or thrombectomy were compared based on the initial dispatch (both MSU and conventional ambulance or conventional ambulance only).
Exposure: Simultaneous dispatch of an MSU (computed tomographic scanning with or without angiography, point-of-care laboratory testing, and thrombolysis capabilities on board) and a conventional ambulance (n = 749) vs conventional ambulance alone (n = 794).
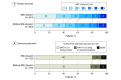
Main outcomes and measures: The primary outcome was the distribution of modified Rankin Scale (mRS) scores (a disability score ranging from 0, no neurological deficits, to 6, death) at 3 months. The coprimary outcome was a 3-tier disability scale at 3 months (none to moderate disability; severe disability; death) with tier assignment based on mRS scores if available or place of residence if mRS scores were not available. Common odds ratios (ORs) were used to quantify the association between exposure and outcome; values less than 1.00 indicated a favorable shift in the mRS distribution and lower odds of higher levels of disability.
Results: Of the 1543 patients (mean age, 74 years; 723 women [47%]) included in the adjusted primary analysis, 1337 (87%) had available mRS scores (primary outcome) and 1506 patients (98%) had available the 3-tier disability scale assessment (coprimary outcome). Patients with an MSU dispatched had lower median mRS scores at month 3 (1; interquartile range [IQR], 0-3) than did patients without an MSU dispatched (2; IQR, 0-3; common OR for worse mRS, 0.71; 95% CI, 0.58-0.86; P < .001). Similarly, patients with an MSU dispatched had lower 3-month coprimary disability scores: 586 patients (80.3%) had none to moderate disability; 92 (12.6%) had severe disability; and 52 (7.1%) had died vs patients without an MSU dispatched: 605 (78.0%) had none to moderate disability; 103 (13.3%) had severe disability; and 68 (8.8%) had died (common OR for worse functional outcome, 0.73, 95% CI, 0.54-0.99; P = .04).
Conclusions and relevance: In this prospective, nonrandomized, controlled intervention study of patients with acute ischemic stroke in Berlin, Germany, the dispatch of mobile stroke units, compared with conventional ambulances alone, was significantly associated with lower global disability at 3 months. Clinical trials in other regions are warranted.
Conflict of interest statement
Figures
Comment in
-
Improving Stroke Treatment and Outcomes With Mobile Stroke Units.JAMA. 2021 Feb 2;325(5):441-442. doi: 10.1001/jama.2020.25832. JAMA. 2021. PMID: 33528517 No abstract available.
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic Stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

