Durability of Complete Response to Intralesional Interleukin-2 for In-Transit Melanoma
- PMID: 33529083
- PMCID: PMC8311908
- DOI: 10.1177/1203475420988862
Durability of Complete Response to Intralesional Interleukin-2 for In-Transit Melanoma
Abstract
Background: Intralesional injection of interleukin-2 (IL-2) for in-transit melanoma (ITM) is associated with a high rate of complete response. However, there is a paucity of data on treatment durability and long-term outcomes.
Objectives: To provide long-term data on patients with a complete response to IL-2 therapy for ITM.
Methods: Consecutive patients with ITM, treated with intralesional IL-2 therapy, at the Tom Baker Cancer Center were identified from April 2009 to August 2019. All patients received at least 4 cycles (every 2 weeks) of IL-2 (5 MIU/mL). Complete response was defined as sustained (ie, 3 months) clinical complete remission of all known in-transit disease.
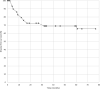
Results: Sixty-five patients were treated with curative intent for in-transit disease with intralesional IL-2. Complete clinical response was identified in 44.6% (29/65). In this subset of patients, the median number of lesions per patient was 9 (range 1-40). The median total dose of IL-2 was 0.8 mL (IQR 0.4-1.5) per lesion. One patient received isolated limb infusion and 13.8% (4/29) received systemic immunotherapy as part of their initial management. At a median follow-up of 27 months (IQR 16-59), 34.5% (10/29) developed recurrent disease. Of these patients, 50.0% (5/10) presented with synchronous in-transit and distant metastases. The median time to recurrence was 10.5 months (IQR 5.8-16.3).
Conclusion: With long-term follow-up, 65.5% of complete responders have a durable response to intralesional IL-2 therapy. In this cohort of patients, local in-transit recurrence is most likely to occur within 12 months and is often associated with concomitant distant disease.
Keywords: in-transit melanoma; intralesional interleukin-2 (IL-2); recurrence.
Conflict of interest statement
Figures
References
-
- Canadian Cancer Statistics Advisory Committee . Canadian Cancer Statistics 2019. Toronto, ON: Canadian Cancer Society; 2019. Accessed May 5, 2020. cancer.ca/Canadian-Cancer-Statistics-2019-EN
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous