Development of a coronavirus disease 2019 nonhuman primate model using airborne exposure
- PMID: 33529233
- PMCID: PMC7853502
- DOI: 10.1371/journal.pone.0246366
Development of a coronavirus disease 2019 nonhuman primate model using airborne exposure
Abstract
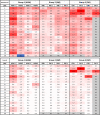
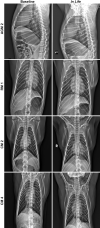
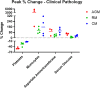
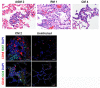
Airborne transmission is predicted to be a prevalent route of human exposure with SARS-CoV-2. Aside from African green monkeys, nonhuman primate models that replicate airborne transmission of SARS-CoV-2 have not been investigated. A comparative evaluation of COVID-19 in African green monkeys, rhesus macaques, and cynomolgus macaques following airborne exposure to SARS-CoV-2 was performed to determine critical disease parameters associated with disease progression, and establish correlations between primate and human COVID-19. Respiratory abnormalities and viral shedding were noted for all animals, indicating successful infection. Cynomolgus macaques developed fever, and thrombocytopenia was measured for African green monkeys and rhesus macaques. Type II pneumocyte hyperplasia and alveolar fibrosis were more frequently observed in lung tissue from cynomolgus macaques and African green monkeys. The data indicate that, in addition to African green monkeys, macaques can be successfully infected by airborne SARS-CoV-2, providing viable macaque natural transmission models for medical countermeasure evaluation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Lu S, Zhao Y, Yu W, Yang Y, Gao J, Wang J, et al. Comparison of SARS-CoV-2 infections among 3 species of non-human primates. bioRxiv. 2020. Epub 2020/04/08. 10.1101/2020.04.08.031807 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

