Tyrosine kinase inhibitor associated vascular toxicity in chronic myeloid leukemia
- PMID: 33530148
- PMCID: PMC7837152
- DOI: 10.1186/s40959-015-0008-5
Tyrosine kinase inhibitor associated vascular toxicity in chronic myeloid leukemia
Abstract
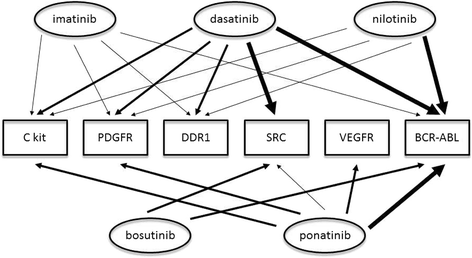
Tyrosine kinase inhibitors (TKIs) have revolutionized the management and outcomes of chronic myeloid leukemia (CML) patients. Improved disease control and prolonged life expectancy now mandate focus on improving TKIs' safety profile. Recently, vascular adverse events (VAEs) have emerged as a serious consequence of some of the newer TKIs. In this review, we describe the clinical spectrum of TKI-associated VAE, and examine the unique vascular safety profile of the main TKIs currently used in the treatment of CML: imatinib, nilotinib, dasatinib, bosutinib and ponatinib. The issue of TKI-related platelet dysfunction is discussed as well. We describe the contemporary research findings regarding the possible pathogenesis of the VAE. Finally, the different aspects of TKI-associated VAE management are addressed, including prevention methods, monitoring strategies and treatment options.
Keywords: Chronic myeloid leukemia; Nilotinib; Platelets; Ponatinib; Tyrosine kinase inhibitor; Vascular adverse events.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
