Single-Fraction Adjuvant Electronic Brachytherapy after Resection of Conjunctival Carcinoma
- PMID: 33530293
- PMCID: PMC7865874
- DOI: 10.3390/cancers13030454
Single-Fraction Adjuvant Electronic Brachytherapy after Resection of Conjunctival Carcinoma
Abstract
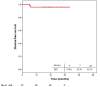
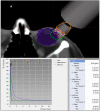
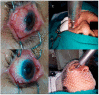
A retrospective study was performed to assess the outcomes of a single-fraction adjuvant electronic brachytherapy (e-BT) approach for patients with squamous cell conjunctival carcinoma (SCCC). Forty-seven patients with T1-T3 SCCC were included. All patients underwent surgery followed by a single-fraction adjuvant e-BT with a porTable 50-kV device. Depending on margins, e-BT doses ranged between 18 to 22 Gy prescribed at 2 mm depth, resembling equivalent doses in 2 Gy (EQD2) per fraction of 46-66 Gy (α/β ratio of 8-10 Gy and a relative biological effect (RBE) of 1.3). The median age was 69 (29-87) years. Most tumors were T1 (40.4%) or T2 (57.5%) with a median size of 7 mm (1.5-20). Margins were positive in 40.4% of cases. The median time from surgery to e-BT was nine weeks (0-37). After a median follow-up of 24 (17-40) months, recurrence occurred in only two patients (6 and 7 months after e-BT), yielding a median disease-free survival (DFS) of 24 (6-40) months and DFS at two years of 95.7%. Acute grade 2 conjunctivitis occurred in 25.5%. E-BT is a safe and effective for SCCC treatment, with clinical and logistic advantages compared to classical methods. Longer follow-up and prospective assessment are warranted.
Keywords: conjunctival carcinoma; electronic brachytherapy; kilovoltage; low-energy X-rays; single-fraction brachytherapy.
Conflict of interest statement
G.R.S.: Grants and personal fees from Carl Zeiss Meditec AG, outside of the submitted work. S.S.: Nothing to disclose. M.B.: Nothing to disclose. D.R.: Nothing to disclose. P.F.R.: Nothing to disclose. F.A.G.: reports other from Implacit GmbH, non-financial support from Oncare GmbH, grants and personal fees from NOXXON Pharma AG, grants and personal fees from Carl Zeiss Meditec AG, personal fees from Bristol-Myers Squibb, personal fees from Roche Pharma AG, personal fees from MSD Sharp and Dohme GmbH, and personal fees from AstraZeneca GmbH, outside the submitted work. In addition, F.A.G. has a patent (US 62/435405) pending. G.J.S.: has received grants and personal fees from Carl Zeiss Meditec AG, outside of the submitted work.
Figures
References
-
- Shields C.L., Alset A.E., Boal N.S., Casey M.G., Knapp A.N., Sugarman J.A., Schoen M.A., Gordon P.S., Douglass A.M., Sioufi K., et al. Conjunctival Tumors in 5002 Cases. Comparative Analysis of Benign Versus Malignant Counterparts. The 2016 James, D. Allen Lecture. Am. J. Ophthalmol. 2017;173:106–133. doi: 10.1016/j.ajo.2016.09.034. - DOI - PubMed
-
- Sun E.C., Fears T.R., Goedert J.J. Epidemiology of squamous cell conjunctival cancer. Cancer Epidemiol. Biomark. Prev. 1997;6:73–77. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

