Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light
- PMID: 33530294
- PMCID: PMC7866012
- DOI: 10.3390/ijms22031184
Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light
Abstract
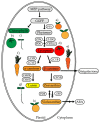
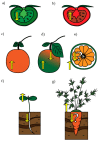
Light is an important cue that stimulates both plastid development and biosynthesis of carotenoids in plants. During photomorphogenesis or de-etiolation, photoreceptors are activated and molecular factors for carotenoid and chlorophyll biosynthesis are induced thereof. In fruits, light is absorbed by chloroplasts in the early stages of ripening, which allows a gradual synthesis of carotenoids in the peel and pulp with the onset of chromoplasts' development. In roots, only a fraction of light reaches this tissue, which is not required for carotenoid synthesis, but it is essential for root development. When exposed to light, roots start greening due to chloroplast development. However, the colored taproot of carrot grown underground presents a high carotenoid accumulation together with chromoplast development, similar to citrus fruits during ripening. Interestingly, total carotenoid levels decrease in carrots roots when illuminated and develop chloroplasts, similar to normal roots exposed to light. The recent findings of the effect of light quality upon the induction of molecular factors involved in carotenoid synthesis in leaves, fruit, and roots are discussed, aiming to propose consensus mechanisms in order to contribute to the understanding of carotenoid synthesis regulation by light in plants.
Keywords: carotenoids; chloroplasts; chromoplasts; light signaling; photoreceptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ram S., Mitra M., Shah F., Tirkey S.R., Mishra S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods. 2020;67:1038673. doi: 10.1016/j.jff.2020.103867. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

