Evaluating the Role of Circulating Dendritic Cells in Methimazole-Treated Pediatric Graves' Disease Patients
- PMID: 33530368
- PMCID: PMC7911035
- DOI: 10.3390/genes12020164
Evaluating the Role of Circulating Dendritic Cells in Methimazole-Treated Pediatric Graves' Disease Patients
Abstract
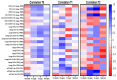
Graves' disease (GD) is hyperthyroidism associated with organ-specific autoimmune inflammation. GD occurs more frequently in adults than in children; however, pediatric patients are a therapeutic challenge due to cycles of remissions and relapses requiring constant monitoring at every stage of treatment administered. Dendritic cells (DCs) are considered to be a link between innate and adaptive immunity. DCs, as antigen-presenting cells (APCs), are involved in antigen presentation to T lymphocytes, thereby initiating a shift towards effector cells. In accordance, DCs also participate in the modulation of tolerance to specific antigens. To date, the data on DCs' role in Graves' pathological processes are scarce. Therefore, here, we evaluated the frequencies and role of circulating DCs in GD pediatric patients treated with methimazole. Flow cytometric analysis was implemented to evaluate three subsets of dendritic cells and their correlation with clinical GD-related parameters. We found significantly higher levels of DC subsets in patients at diagnosis. Furthermore, methimazole treatment seemed to effectively reduce subsets of DCs, which, in addition, were found to differentially correlate with thyroid function. Our study shed new light on DCs' role in the pediatric GD pathomechanism. Further studies are required for the mechanistic assessment of DCs' exact role in disease progression and influence on thyroid function.
Keywords: Graves’ disease; autoimmunity; dendritic cells; methimazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

