The Effects of Association of Topical Polydatin Improves the Preemptive Systemic Treatment on EGFR Inhibitors Cutaneous Adverse Reactions
- PMID: 33530427
- PMCID: PMC7866016
- DOI: 10.3390/jcm10030466
The Effects of Association of Topical Polydatin Improves the Preemptive Systemic Treatment on EGFR Inhibitors Cutaneous Adverse Reactions
Abstract
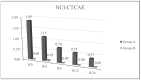
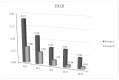
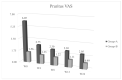
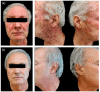
Epidermal Growth Factor Receptor inhibitors (EGFRi) are approved as therapeutic options in several solid tumors. Cutaneous papulopustular eruption is the most frequent cutaneous adverse-event (AE), usually treated with emollient or corticosteroids according to toxicity grade. Our study evaluated the efficacy and safety of a topical product containing polydatin, a glycosylated polyphenol, natural precursor of resveratrol showing anti-inflammatory and anti-oxidative activities, for the prevention and treatment of skin papulopustular rash in EGFRi-treated patients. Forty oncologic patients treated with EGFRi were enrolled in two groups: group-A, 20 patients with papulopustular AE, and group-B, 20 patients without cutaneous manifestations. The study consisted of twice-daily application of polydatin cream 1.5% (group-A) and 0.8% (group-B) for 6 months. In group-A patients, we observed at week 4 a remarkable improvement of skin manifestation and quality of life evaluated with National-Cancer-Institute-Common-Terminology-Criteria for Adverse-Events (NCI-CTCAE), Dermatology-Life-Quality-Index (DLQI) score and Visual-Analogue-Scale (VAS) pruritus, with a statistical significance of p < 0.05. None of the patients of group-B developed skin AEs to EGFRi. No cutaneous AEs related to the polydatin product were reported in both groups. Polydatin can be a good topical aid for the prevention and management of papulopustular rash in cancer patients receiving EGFRi, also capable of improving cancer patients' quality of life.
Keywords: EGFR inhibitors; cutaneous adverse events; papulopustular rash; polydatin.
Conflict of interest statement
The authors declare no conflict of interest. The funders (institutional grant) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Wang Z. ErbB Receptors and Cancer. Methods Mol. Biol. 2017;1652:3–35. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

