The Role of Csmd1 during Mammary Gland Development
- PMID: 33530646
- PMCID: PMC7912059
- DOI: 10.3390/genes12020162
The Role of Csmd1 during Mammary Gland Development
Abstract
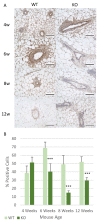
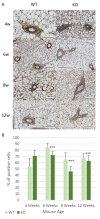
The Cub Sushi Multiple Domains-1 (CSMD1) protein is a tumour suppressor which has been shown to play a role in regulating human mammary duct development in vitro. CSMD1 knockdown in vitro demonstrated increased cell proliferation, invasion and motility. However, the role of Csmd1 in vivo is poorly characterised when it comes to ductal development and is therefore an area which warrants further exploration. In this study a Csmd1 knockout (KO) mouse model was used to identify the role of Csmd1 in regulating mammary gland development during puberty. Changes in duct development and protein expression patterns were analysed by immunohistochemistry. This study identified increased ductal development during the early stages of puberty in the KO mice, characterised by increased ductal area and terminal end bud number at 6 weeks. Furthermore, increased expression of various proteins (Stat1, Fak, Akt, Slug/Snail and Progesterone receptor) was shown at 4 weeks in the KO mice, followed by lower expression levels from 6 weeks in the KO mice compared to the wild type mice. This study identifies a novel role for Csmd1 in mammary gland development, with Csmd1 KO causing significantly more rapid mammary gland development, suggesting an earlier adult mammary gland formation.
Keywords: Akt; Csmd1; Fak; Stat1; epithelial mesenchymal transition; extracellular matrix; mammary gland development; terminal end bud.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Toomes C., Jackson A., Maguire K., Wood J., Gollin S., Ishwad C., Paterson I., Prime S., Parkinson K., Bell S., et al. The Presence of Multiple Regions of Homozygous Deletion at the CSMD1 Locus in Oral Squamous Cell Carcinoma Question the Role of CSMD1 in Head and Neck Carcinogenesis. Genes. Chromosomes Cancer. 2003;37:132–140. doi: 10.1002/gcc.10191. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

