BCL11B Regulates Arterial Stiffness and Related Target Organ Damage
- PMID: 33530702
- PMCID: PMC7969164
- DOI: 10.1161/CIRCRESAHA.120.316666
BCL11B Regulates Arterial Stiffness and Related Target Organ Damage
Abstract
[Figure: see text].
Keywords: actins; blood pressure; calcineurin; phosphorylation; vascular smooth muscle; vascular stiffness.
Figures
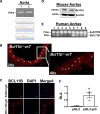




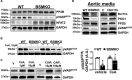


Comment in
-
Targeting Cell Stiffness: A Paradigm Shift in the Treatment of Aortic Stiffness.Circ Res. 2021 Mar 19;128(6):769-771. doi: 10.1161/CIRCRESAHA.121.318954. Epub 2021 Mar 18. Circ Res. 2021. PMID: 33734817 No abstract available.
References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596 - PubMed
-
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342 - PubMed
-
- Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065 - PMC - PubMed
-
- AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

