Title: insoluble proteins catch heterologous soluble proteins into inclusion bodies by intermolecular interaction of aggregating peptides
- PMID: 33531005
- PMCID: PMC7852131
- DOI: 10.1186/s12934-021-01524-3
Title: insoluble proteins catch heterologous soluble proteins into inclusion bodies by intermolecular interaction of aggregating peptides
Abstract
Background: Protein aggregation is a biological event observed in expression systems in which the recombinant protein is produced under stressful conditions surpassing the homeostasis of the protein quality control system. In addition, protein aggregation is also related to conformational diseases in animals as transmissible prion diseases or non-transmissible neurodegenerative diseases including Alzheimer, Parkinson's disease, amyloidosis and multiple system atrophy among others. At the molecular level, the presence of aggregation-prone domains in protein molecules act as seeding igniters to induce the accumulation of protein molecules in protease-resistant clusters by intermolecular interactions.
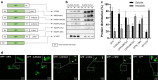
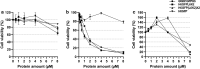
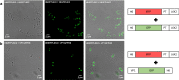
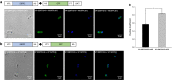
Results: In this work we have studied the aggregating-prone performance of a small peptide (L6K2) with additional antimicrobial activity and we have elucidated the relevance of the accompanying scaffold protein to enhance the aggregating profile of the fusion protein. Furthermore, we demonstrated that the fusion of L6K2 to highly soluble recombinant proteins directs the protein to inclusion bodies (IBs) in E. coli through stereospecific interactions in the presence of an insoluble protein displaying the same aggregating-prone peptide (APP).
Conclusions: These data suggest that the molecular bases of protein aggregation are related to the net balance of protein aggregation potential and not only to the presence of APPs. This is then presented as a generic platform to generate hybrid protein aggregates in microbial cell factories for biopharmaceutical and biotechnological applications.
Keywords: Antimicrobial peptides; Inclusion body formation; Intermolecular interaction; Protein aggregation; Recombinant protein.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem. 2017;86:27–68. - PubMed
-
- Dannies PS. Prolactin and growth hormone aggregates in secretory granules: the need to understand the structure of the aggregate. Endocr Rev. 2012;33:254–70. - PubMed
-
- Corchero JL. Eukaryotic aggresomes: from a model of conformational diseases to an emerging type of immobilized biocatalyzers. Appl Microbiol Biotechnol. 2016;100:559–69. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

