Plasma membrane H+-ATPase overexpression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis
- PMID: 33531490
- PMCID: PMC7854686
- DOI: 10.1038/s41467-021-20964-4
Plasma membrane H+-ATPase overexpression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis
Abstract
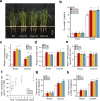
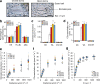
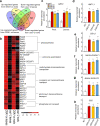
Nitrogen (N) and carbon (C) are essential elements for plant growth and crop yield. Thus, improved N and C utilisation contributes to agricultural productivity and reduces the need for fertilisation. In the present study, we find that overexpression of a single rice gene, Oryza sativa plasma membrane (PM) H+-ATPase 1 (OSA1), facilitates ammonium absorption and assimilation in roots and enhanced light-induced stomatal opening with higher photosynthesis rate in leaves. As a result, OSA1 overexpression in rice plants causes a 33% increase in grain yield and a 46% increase in N use efficiency overall. As PM H+-ATPase is highly conserved in plants, these findings indicate that the manipulation of PM H+-ATPase could cooperatively improve N and C utilisation, potentially providing a vital tool for food security and sustainable agriculture.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants (Elsevier, 2012).
-
- Smil V. Detonator of the population explosion. Nature. 1999;400:415. doi: 10.1038/22672. - DOI
-
- Omar P, Aula L, Oyebiyi F, Raun WR. World cereal nitrogen use efficiency trends: review and current knowledge. Agrosyst. Geosci. Environ. 2019;2:180045.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

