Automated digital TIL analysis (ADTA) adds prognostic value to standard assessment of depth and ulceration in primary melanoma
- PMID: 33531581
- PMCID: PMC7854647
- DOI: 10.1038/s41598-021-82305-1
Automated digital TIL analysis (ADTA) adds prognostic value to standard assessment of depth and ulceration in primary melanoma
Abstract
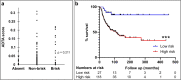
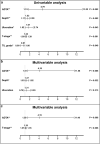
Accurate prognostic biomarkers in early-stage melanoma are urgently needed to stratify patients for clinical trials of adjuvant therapy. We applied a previously developed open source deep learning algorithm to detect tumor-infiltrating lymphocytes (TILs) in hematoxylin and eosin (H&E) images of early-stage melanomas. We tested whether automated digital (TIL) analysis (ADTA) improved accuracy of prediction of disease specific survival (DSS) based on current pathology standards. ADTA was applied to a training cohort (n = 80) and a cutoff value was defined based on a Receiver Operating Curve. ADTA was then applied to a validation cohort (n = 145) and the previously determined cutoff value was used to stratify high and low risk patients, as demonstrated by Kaplan-Meier analysis (p ≤ 0.001). Multivariable Cox proportional hazards analysis was performed using ADTA, depth, and ulceration as co-variables and showed that ADTA contributed to DSS prediction (HR: 4.18, CI 1.51-11.58, p = 0.006). ADTA provides an effective and attainable assessment of TILs and should be further evaluated in larger studies for inclusion in staging algorithms.
Conflict of interest statement
Dr. Saenger has received research funding from Amgen and Regeneron. In addition, she is co-founder of a small biomarkers start-up company, Wasaba. All other authors declare no competing interests.
Figures


References
-
- Weber J, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

