Systemic corticosteroid use in UK Uveitis practice: results from the ocular inflammation steroid toxicity risk (OSTRICH) study
- PMID: 33531696
- PMCID: PMC8602319
- DOI: 10.1038/s41433-020-01336-6
Systemic corticosteroid use in UK Uveitis practice: results from the ocular inflammation steroid toxicity risk (OSTRICH) study
Abstract
Objectives: To ascertain adherence to an international consensus target of ≤7.5 mg/day of prednisolone for maintenance systemic corticosteroid (CS) prescribing in uveitis and report the frequency of courses of high-dose systemic CS in the UK.
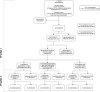
Methods: We conducted a national, multicentre audit of systemic CS prescribing for uveitis at 11 UK sites between November 2018 and March 2019. High-dose CS was defined as (1) maintenance >7.5 mg prednisolone for >3 consecutive months, or (2) >1 course ≥40 mg oral CS or ≥500 mg intravenous (IV) methylprednisolone in the past 12 months. Case notes of patients exceeding threshold CS doses were reviewed by an independent uveitis specialist and judged as avoidable or not, based upon a scoring matrix.
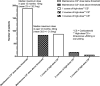
Results: Of 667 eligible patients, 285 (42.7%) were treated with oral or IV CS over the preceding 12 months; 96 (33.7%) of these exceeded the threshold for high-dose CS. Twenty-five percent of prescribing in patients on excess CS was judged avoidable; attributed to either prescribing long-term CS without evidence of consideration of alternative strategies, prescribing error or miscommunication. More patients received immunomodulatory therapy (IMT) in the group treated with CS above threshold than below threshold (p < 0.001) but there was no significant difference in doses of IMT.
Conclusion: 33% of patients had been prescribed excessive corticosteroid when compared to the reference standard. An analysis of decision-making suggests there may be opportunity to reduce excess CS prescribing in 25% of these patients.
© 2021. The Author(s), under exclusive licence to The Royal College of Ophthalmologists.
Conflict of interest statement
SMS: Paid advisory boards for Alimera Sciences, Gilead, Abbvie and Allergan; NB: paid advisory boards for Alimera Sciences and Gilead. NB’s institution has received funds from Allergan.
Figures
Comment in
-
Comment on: Systemic corticosteroid use in UK Uveitis practice: results from the ocular inflammation steroid toxicity risk (OSTRICH) study.Eye (Lond). 2022 Nov;36(11):2223. doi: 10.1038/s41433-022-02031-4. Epub 2022 Mar 23. Eye (Lond). 2022. PMID: 35322212 Free PMC article. No abstract available.
References
-
- Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004. 10.7326/0003-4819-141-10-200411160-00007. - PubMed
-
- Kremer JM. The CORRONA database. Autoimmun Rev. 2006. 10.1016/j.autrev.2005.07.006. - PubMed
-
- Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am J Ophthalmol. 2000. 10.1016/S0002-9394(00)00659-0. - PubMed
-
- Dick AD, Rosenbaum JT, Al-Dhibi HA, et al. Guidance on Noncorticosteroid Systemic Immunomodulatory Therapy in Noninfectious Uveitis: Fundamentals Of Care for UveitiS (FOCUS) Initiative. Ophthalmology. 2018. 10.1016/j.ophtha.2017.11.017. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

