Variation in SARS-CoV-2 outbreaks across sub-Saharan Africa
- PMID: 33531710
- PMCID: PMC8590469
- DOI: 10.1038/s41591-021-01234-8
Variation in SARS-CoV-2 outbreaks across sub-Saharan Africa
Abstract
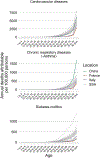
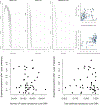
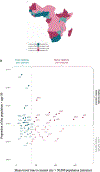
A surprising feature of the SARS-CoV-2 pandemic to date is the low burdens reported in sub-Saharan Africa (SSA) countries relative to other global regions. Potential explanations (for example, warmer environments1, younger populations2-4) have yet to be framed within a comprehensive analysis. We synthesized factors hypothesized to drive the pace and burden of this pandemic in SSA during the period from 25 February to 20 December 2020, encompassing demographic, comorbidity, climatic, healthcare capacity, intervention efforts and human mobility dimensions. Large diversity in the probable drivers indicates a need for caution in interpreting analyses that aggregate data across low- and middle-income settings. Our simulation shows that climatic variation between SSA population centers has little effect on early outbreak trajectories; however, heterogeneity in connectivity, although rarely considered, is likely an important contributor to variance in the pace of viral spread across SSA. Our synthesis points to the potential benefits of context-specific adaptation of surveillance systems during the ongoing pandemic. In particular, characterizing patterns of severity over age will be a priority in settings with high comorbidity burdens and poor access to care. Understanding the spatial extent of outbreaks warrants emphasis in settings where low connectivity could drive prolonged, asynchronous outbreaks resulting in extended stress to health systems.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures







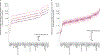




Update of
-
High variation expected in the pace and burden of SARS-CoV-2 outbreaks across sub-Saharan Africa.medRxiv [Preprint]. 2020 Jul 29:2020.07.23.20161208. doi: 10.1101/2020.07.23.20161208. medRxiv. 2020. Update in: Nat Med. 2021 Mar;27(3):447-453. doi: 10.1038/s41591-021-01234-8. PMID: 32743598 Free PMC article. Updated. Preprint.
References
-
- Rinaldi G & Paradisi M An empirical estimate of the infection fatality rate of COVID-19 from the first Italian outbreak. Preprint at medRxiv 10.1101/2020.04.18.20070912 (2020). - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

