Response Surface Optimization of Ultra-Elastic Nanovesicles Loaded with Deflazacort Tailored for Transdermal Delivery: Accentuated Bioavailability and Anti-Inflammatory Efficacy
- PMID: 33531803
- PMCID: PMC7846863
- DOI: 10.2147/IJN.S276330
Response Surface Optimization of Ultra-Elastic Nanovesicles Loaded with Deflazacort Tailored for Transdermal Delivery: Accentuated Bioavailability and Anti-Inflammatory Efficacy
Abstract
Purpose: The aim of the present study was to develop deflazacort (DFZ) ultra-elastic nanovesicles (UENVs) loaded gel for topical administration to evade gastrointestinal adverse impacts accompanying DFZ oral therapy.
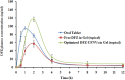
Methods: UENVs were elaborated according to D-optimal mixture design employing different edge activators as Span-60, Tween-85 and sodium cholate which were incorporated into the nanovesicles to improve the deformability of vesicles bilayer. DFZ-UENVs were formulated by thin-film hydration technique followed by characterization for different parameters including entrapment efficiency (%EE), particle size, in vitro release and ex vivo permeation studies. The composition of the optimized DFZ-UENV formulation was found to be DFZ (10 mg), Span-60 (30 mg), Tween-85 (30 mg), sodium cholate (3.93 mg), L-α phosphatidylcholine (60 mg) and cholesterol (30 mg). The optimum formulation was incorporated into hydrogel base then characterized in terms of physical parameters, in vitro drug release, ex vivo permeation study and pharmacodynamics evaluation. Finally, pharmacokinetic study in rabbits was performed via transdermal application of UENVs gel in comparison to oral drug.
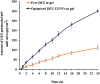
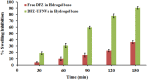
Results: The optimum UENVs formulation exhibited %EE of 74.77±1.33, vesicle diameter of 219.64±2.52 nm, 68.88±1.64% of DFZ released after 12 h and zeta potential of -55.57±1.04 mV. The current work divulged successful augmentation of the bioavailability of DFZ optimum formulation by about 1.37-fold and drug release retardation compared to oral drug tablets besides significant depression of edema, cellular inflammation and capillary congestion in carrageenan-induced rat paw edema model.
Conclusion: The transdermal DFZ-UENVs can achieve boosted bioavailability and may be suggested as an auspicious non-invasive alternative platform for oral route.
Keywords: D-optimal design; deflazacort; pharmacodynamics; pharmacokinetics; ultra-elastic nanovesicles.
© 2021 Ali et al.
Conflict of interest statement
The authors report no conflicts of interest for this work.
Figures


References
-
- Corrêa G, Bellé LP, Bajerski L, et al. Development and validation of a reversed-phase HPLC method for the determination of deflazacort in pharmaceutical dosage forms. Chromatographia. 2007;65(9–10):591–594. doi: 10.1365/s10337-007-0205-y - DOI
-
- Scremin A, Piazzon M, Silva MAS, et al. Spectrophotometric and HPLC determination of deflazacort in pharmaceutical dosage forms. Brazilian J Pharmaceutical Sci. 2010;46:281–287. doi: 10.1590/S1984-82502010000200015 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

