IL-1 Receptor Antagonist Protects the Osteogenesis Capability of Gingival-Derived Stem/Progenitor Cells under Inflammatory Microenvironment Induced by Porphyromonas gingivalis Lipopolysaccharides
- PMID: 33531908
- PMCID: PMC7834827
- DOI: 10.1155/2021/6638575
IL-1 Receptor Antagonist Protects the Osteogenesis Capability of Gingival-Derived Stem/Progenitor Cells under Inflammatory Microenvironment Induced by Porphyromonas gingivalis Lipopolysaccharides
Abstract
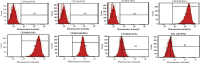
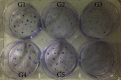
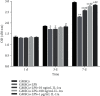
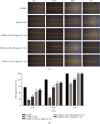
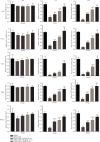
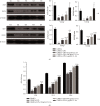
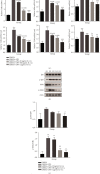
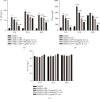
Mesenchymal stem cells (MSCs) have been considered to be a future treatment option for periodontitis due to their excellent regenerative capability. However, it is still a challenge to protect MSCs' biological properties from multiple bacterial toxins in local inflammatory environment. The present study is aimed at investigating the treatment effect of interleukin-1 receptor antagonist (IL-1ra) on cell proliferation, migration, and osteogenic differentiation of gingival-derived mesenchymal stem cells (GMSCs) under an inflammatory microenvironment induced by Porphyromonas gingivalis lipopolysaccharides (P. gingivalis-LPS). GMSCs derived from Sprague-Dawley (SD) rats' free gingival tissues were treated with P. gingivalis-LPS (10 μg/mL) to create in vitro inflammatory environment. Different concentrations of IL-1ra (0.01-1 μg/mL) were used to antagonize the negative effect of LPS. Cell behaviors including proliferation, cloning formation unit (CFU), cell migration, osteogenic differentiation, mineral deposition, and cytokine production were assessed to investigate the protection effect of IL-1ra on GMSCs under inflammation. The toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) pathway activated by LPS was evaluated by real-time quantitative polymerase chain reaction (RT-PCR) and western blot. In response to P. gingivalis-LPS treatment, cell numbers, cloning formation rate, cell migration rate, proinflammatory cytokine production, and osteogenic differentiation-associated protein/mRNA expressions as well as mineralized nodules were suppressed in a time-dependent manner. These negative effects were effectively attenuated by IL-1ra administration in a time- and dose-dependent manner. In addition, mRNA expressions of TLR4 and IkBα decreased dramatically when IL-1ra was added into LPS-induced medium. IL-1ra also reversed the LPS-induced TLR4/NF-κB activation as indicated by western blot. The present study revealed that IL-1ra decreased inflammatory cytokine production in a supernatant, so as to protect GMSCs' osteogenesis capacity and other biological properties under P. gingivalis-LPS-induced inflammatory environment. This might be explained by IL-1ra downregulating TLR4-mediated NF-κB signaling pathway activation.
Copyright © 2021 Yuxin Zhao et al.
Conflict of interest statement
The authors declare no conflict of interest regarding the publication of this article.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

