Metabolomics study reveals the potential evidence of metabolic reprogramming towards the Warburg effect in precancerous lesions
- PMID: 33532002
- PMCID: PMC7847643
- DOI: 10.7150/jca.54252
Metabolomics study reveals the potential evidence of metabolic reprogramming towards the Warburg effect in precancerous lesions
Abstract
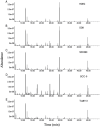
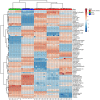
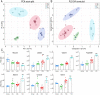
Background: Most tumors have an enhanced glycolysis flux, even when oxygen is available, called the aerobic glycolysis or the Warburg effect. Metabolic reprogramming promotes cancer progression, and is even related to the tumorigenesis. However, it is not clear whether the observed metabolic changes act as a driver or a bystander in cancer development. Methods: In this study, the metabolic characteristics of oral precancerous cells and cervical precancerous lesions were analyzed by metabolomics, and the expression of glycolytic enzymes in cervical precancerous lesions was evaluated by RT-PCR and Western blot analysis. Results: In total, 115 and 23 metabolites with reliable signals were identified in oral cells and cervical tissues, respectively. Based on the metabolome, oral precancerous cell DOK could be clearly separated from normal human oral epithelial cells (HOEC) and oral cancer cells. Four critical differential metabolites (pyruvate, glutamine, methionine and lysine) were identified between DOK and HOEC. Metabolic profiles could clearly distinguish cervical precancerous lesions from normal cervical epithelium and cervical cancer. Compared with normal cervical epithelium, the glucose consumption and lactate production increased in cervical precancerous lesions. The expression of glycolytic enzymes LDHA, HK II and PKM2 showed an increased tendency in cervical precancerous lesions compared with normal cervical epithelium. Conclusions: Our findings suggest that cell metabolism may be reprogrammed at the early stage of tumorigenesis, implying the contribution of metabolic reprogramming to the development of tumor.
Keywords: glycolytic enzymes; metabolic reprogramming; metabolomics; precancerous lesions; the Warburg-like effect.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Signal transducer and activator of transcription 3 promotes the Warburg effect possibly by inducing pyruvate kinase M2 phosphorylation in liver precancerous lesions.World J Gastroenterol. 2019 Apr 28;25(16):1936-1949. doi: 10.3748/wjg.v25.i16.1936. World J Gastroenterol. 2019. PMID: 31086462 Free PMC article.
-
The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression.Int J Radiat Biol. 2019 Jul;95(7):912-919. doi: 10.1080/09553002.2019.1589653. Epub 2019 Mar 22. Int J Radiat Biol. 2019. PMID: 30822194 Review.
-
Metabolic reprogramming in cervical cancer and metabolomics perspectives.Nutr Metab (Lond). 2021 Oct 19;18(1):93. doi: 10.1186/s12986-021-00615-7. Nutr Metab (Lond). 2021. PMID: 34666780 Free PMC article. Review.
-
Caffeic Acid Targets AMPK Signaling and Regulates Tricarboxylic Acid Cycle Anaplerosis while Metformin Downregulates HIF-1α-Induced Glycolytic Enzymes in Human Cervical Squamous Cell Carcinoma Lines.Nutrients. 2018 Jun 28;10(7):841. doi: 10.3390/nu10070841. Nutrients. 2018. PMID: 29958416 Free PMC article.
-
Tyrosine Kinase Signaling in Cancer Metabolism: PKM2 Paradox in the Warburg Effect.Front Cell Dev Biol. 2018 Jul 24;6:79. doi: 10.3389/fcell.2018.00079. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30087897 Free PMC article. Review.
Cited by
-
Metabolomic Profiling of Oral Potentially Malignant Disorders and Its Clinical Values.Biomedicines. 2024 Dec 19;12(12):2899. doi: 10.3390/biomedicines12122899. Biomedicines. 2024. PMID: 39767805 Free PMC article. Review.
-
Altered metabolic profiles in colon and rectal cancer.Sci Rep. 2025 Apr 2;15(1):11310. doi: 10.1038/s41598-025-96004-8. Sci Rep. 2025. PMID: 40175601 Free PMC article.
-
Methionine Dependency and Restriction in Cancer: Exploring the Pathogenic Function and Therapeutic Potential.Pharmaceuticals (Basel). 2025 Apr 28;18(5):640. doi: 10.3390/ph18050640. Pharmaceuticals (Basel). 2025. PMID: 40430461 Free PMC article. Review.
-
The essential molecular requirements for the transformation of normal cells into established cancer cells, with implications for a novel anti-cancer agent.Cancer Rep (Hoboken). 2023 Aug;6(8):e1844. doi: 10.1002/cnr2.1844. Epub 2023 Jun 6. Cancer Rep (Hoboken). 2023. PMID: 37279947 Free PMC article. Review.
-
Relationship between vaginal and oral microbiome in patients of human papillomavirus (HPV) infection and cervical cancer.J Transl Med. 2024 Apr 29;22(1):396. doi: 10.1186/s12967-024-05124-8. J Transl Med. 2024. PMID: 38685022 Free PMC article.
References
-
- Chen X, Zhao Y. Human papillomavirus infection in oral potentially malignant disorders and cancer. Arch Oral Biol. 2017;83:334–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

