P2X7 Receptor Induces Pyroptotic Inflammation and Cartilage Degradation in Osteoarthritis via NF- κ B/NLRP3 Crosstalk
- PMID: 33532039
- PMCID: PMC7834826
- DOI: 10.1155/2021/8868361
P2X7 Receptor Induces Pyroptotic Inflammation and Cartilage Degradation in Osteoarthritis via NF- κ B/NLRP3 Crosstalk
Abstract
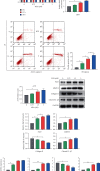
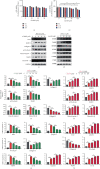
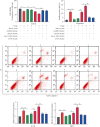
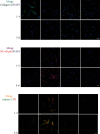
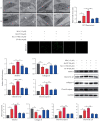
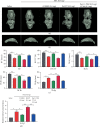
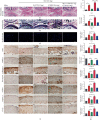
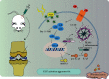
Osteoarthritis (OA) is an urgent public health problem; however, the underlying causal mechanisms remain unclear, especially in terms of inflammatory mediators in cartilage degradation and chondrocyte imbalance. P2X7 receptor (P2X7R) is a critical inflammation switch, but few studies have examined its function and mechanisms in OA-like pyroptotic inflammation of chondrocytes. In this study, Sprague-Dawley rats were injected in the knee with monosodium iodoacetate (MIA) to induce OA, followed by multiple intra-articular injections with P2X7R antagonist A740003, P2X7R agonist BzATP, NF-κB inhibitor Bay 11-7082, and NLRP3 inhibitor CY-09. Primary rat chondrocytes were harvested and treated similarly. We assessed cell viability, damage, and death via cell viability assay, lactate dehydrogenase (LDH) release, and flow cytometry. Concentrations of adenosine triphosphate (ATP) and interleukin- (IL-) 1β in cell culture supernatant and joint cavity lavage fluid were analyzed by enzyme-linked immunosorbent assay. Changes in expression levels of P2X7 and inflammation-related indicators were analyzed by immunofluorescence, quantitative reverse-transcription polymerase chain reaction, and western blotting. Cell morphology changes and pyroptosis were observed using transmission electron microscopy. Histology, immunohistochemistry, and microcomputed tomography were used to analyze damage to bone and cartilage tissues and assess the severity of OA. Similar to MIA, BzATP reduced cell viability and collagen II expression in a dose-dependent manner. Conversely, A740003 ameliorated MIA-induced cartilage degradation and OA-like pyroptotic inflammation by rescuing P2X7, MMP13, NF-κB p65, NLRP3, caspase-1 (TUNEL-positive and active), and IL-1β upregulation. Additionally, A740003 reduced the caspase-1/propidium iodide double-positive rate, LDH concentration, and reactive oxygen species production. These effects also occurred via coincubation with Bay 11-7082 and CY-09. In conclusion, activated P2X7 promoted extracellular matrix degradation and pyroptotic inflammation in OA chondrocytes through NF-κB/NLRP3 crosstalk, thus, aggravating the symptoms of OA. The study findings suggest P2X7 as a potential target for inflammation treatment, providing new avenues for OA research and therapy.
Copyright © 2021 Zihao Li et al.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Blocking of the P2X7 receptor inhibits the activation of the MMP-13 and NF-κB pathways in the cartilage tissue of rats with osteoarthritis.Int J Mol Med. 2016 Dec;38(6):1922-1932. doi: 10.3892/ijmm.2016.2770. Epub 2016 Oct 13. Int J Mol Med. 2016. PMID: 27748894
-
Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis.J Orthop Surg Res. 2019 Sep 11;14(1):307. doi: 10.1186/s13018-019-1307-6. J Orthop Surg Res. 2019. PMID: 31511005 Free PMC article.
-
Activation of the alpha 7 nicotinic acetylcholine receptor mitigates osteoarthritis progression by inhibiting NF-κB/NLRP3 inflammasome activation and enhancing autophagy.PLoS One. 2021 Dec 23;16(12):e0256507. doi: 10.1371/journal.pone.0256507. eCollection 2021. PLoS One. 2021. PMID: 34941874 Free PMC article.
-
Pyroptosis for osteoarthritis treatment: insights into cellular and molecular interactions inflammatory.Front Immunol. 2025 Apr 1;16:1556990. doi: 10.3389/fimmu.2025.1556990. eCollection 2025. Front Immunol. 2025. PMID: 40236711 Free PMC article. Review.
-
P2X7 receptor and the NLRP3 inflammasome: Partners in crime.Biochem Pharmacol. 2021 May;187:114385. doi: 10.1016/j.bcp.2020.114385. Epub 2020 Dec 20. Biochem Pharmacol. 2021. PMID: 33359010 Review.
Cited by
-
Screening chondrocyte necroptosis-related genes in the diagnosis and treatment of osteoarthritis.Heliyon. 2024 Jul 31;10(15):e35263. doi: 10.1016/j.heliyon.2024.e35263. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170298 Free PMC article.
-
Exercise-induced modulation of myokine irisin in bone and cartilage tissue-Positive effects on osteoarthritis: A narrative review.Front Aging Neurosci. 2022 Aug 19;14:934406. doi: 10.3389/fnagi.2022.934406. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36062149 Free PMC article. Review.
-
The regulatory role and therapeutic application of pyroptosis in musculoskeletal diseases.Cell Death Discov. 2022 Dec 15;8(1):492. doi: 10.1038/s41420-022-01282-0. Cell Death Discov. 2022. PMID: 36522335 Free PMC article. Review.
-
NLRP3 Inflammasome: A New Target for Prevention and Control of Osteoporosis?Front Endocrinol (Lausanne). 2021 Sep 27;12:752546. doi: 10.3389/fendo.2021.752546. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34646239 Free PMC article. Review.
-
SCP2 mediates the transport of lipid hydroperoxides to mitochondria in chondrocyte ferroptosis.Cell Death Discov. 2023 Jul 8;9(1):234. doi: 10.1038/s41420-023-01522-x. Cell Death Discov. 2023. PMID: 37422468 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

