Mesenchymal Stem Cell exosome delivered Zinc Finger Protein activation of cystic fibrosis transmembrane conductance regulator
- PMID: 33532041
- PMCID: PMC7825549
- DOI: 10.1002/jev2.12053
Mesenchymal Stem Cell exosome delivered Zinc Finger Protein activation of cystic fibrosis transmembrane conductance regulator
Abstract
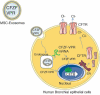
Cystic fibrosis is a genetic disorder that results in a multi-organ disease with progressive respiratory decline which leads to premature death. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene disrupts the capacity of the protein to function as a channel, transporting chloride ions and bicarbonate across epithelial cell membranes. Small molecule treatments targeted at potentiating or correcting CFTR have shown clinical benefits, but are only effective for a small percentage of individuals with specific CFTR mutations. To overcome this limitation, we engineered stromal-derived mesenchymal stem cells (MSC) and HEK293 cells to produce exosomes containing a novel CFTR Zinc Finger Protein fusion with transcriptional activation domains VP64, P65 and Rta to target the CFTR promoter (CFZF-VPR) and activate transcription. Treatment with CFZF-VPR results in robust activation of CFTR transcription in patient derived Human Bronchial Epithelial cells (HuBEC). We also find that CFZF-VPR can be packaged into MSC and HEK293 cell exosomes and delivered to HuBEC cells to potently activate CFTR expression. Connexin 43 appeared to be required for functional release of CFZF-VPR from exosomes. The observations presented here demonstrate that MSC derived exosomes can be used to deliver a packaged zinc finger activator to target cells and activate CFTR. The novel approach presented here offers a next-generation genetic therapy that may one day prove effective in treating patients afflicted with Cystic fibrosis.
Keywords: CFTR; exosome, cystic fibrosis, MSC, Zinc Finger Protein.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
K.V.M and O.V. have submitted a provisional patent on this technology.
Figures



Similar articles
-
Targeted Activation of Cystic Fibrosis Transmembrane Conductance Regulator.Mol Ther. 2019 Oct 2;27(10):1737-1748. doi: 10.1016/j.ymthe.2019.07.002. Epub 2019 Jul 15. Mol Ther. 2019. PMID: 31383454 Free PMC article.
-
Transfer of the Cystic Fibrosis Transmembrane Conductance Regulator to Human Cystic Fibrosis Cells Mediated by Extracellular Vesicles.Hum Gene Ther. 2016 Feb;27(2):166-83. doi: 10.1089/hum.2015.144. Hum Gene Ther. 2016. PMID: 26886833
-
[Cystic fibrosis: new treatments targeting the CFTR protein].Rev Mal Respir. 2013 Apr;30(4):255-61. doi: 10.1016/j.rmr.2012.10.631. Epub 2013 Jan 11. Rev Mal Respir. 2013. PMID: 23664284 Review. French.
-
Respiratory syncytial virus engineered to express the cystic fibrosis transmembrane conductance regulator corrects the bioelectric phenotype of human cystic fibrosis airway epithelium in vitro.J Virol. 2010 Aug;84(15):7770-81. doi: 10.1128/JVI.00346-10. Epub 2010 May 26. J Virol. 2010. PMID: 20504917 Free PMC article.
-
Repairing the basic defect in cystic fibrosis - one approach is not enough.FEBS J. 2016 Jan;283(2):246-64. doi: 10.1111/febs.13531. Epub 2015 Oct 18. FEBS J. 2016. PMID: 26416076 Review.
Cited by
-
Emerging role of exosomes in the pathology of chronic obstructive pulmonary diseases; destructive and therapeutic properties.Stem Cell Res Ther. 2022 Apr 4;13(1):144. doi: 10.1186/s13287-022-02820-4. Stem Cell Res Ther. 2022. PMID: 35379335 Free PMC article. Review.
-
Therapeutic Approaches for Patients with Cystic Fibrosis Not Eligible for Current CFTR Modulators.Cells. 2021 Oct 19;10(10):2793. doi: 10.3390/cells10102793. Cells. 2021. PMID: 34685773 Free PMC article. Review.
-
Astragaloside IV increases PDHA1 in mesenchymal stem cell exosomes to treat myocardial infarction.Sci Rep. 2025 Jul 15;15(1):25461. doi: 10.1038/s41598-025-08628-5. Sci Rep. 2025. PMID: 40659732 Free PMC article.
-
Baohuoside I chemosensitises breast cancer to paclitaxel by suppressing extracellular vesicle/CXCL1 signal released from apoptotic cells.J Extracell Vesicles. 2024 Jul;13(7):e12493. doi: 10.1002/jev2.12493. J Extracell Vesicles. 2024. PMID: 39051750 Free PMC article.
-
Extracellular Vesicles Loaded with Long Antisense RNAs Repress Severe Acute Respiratory Syndrome Coronavirus 2 Infection.Nucleic Acid Ther. 2024;34(3):101-108. doi: 10.1089/nat.2023.0078. Epub 2024 Mar 26. Nucleic Acid Ther. 2024. PMID: 38530082 Free PMC article.
References
-
- Bang, C. , & Thum, T. (2012). Exosomes: new players in cell‐cell communication. International Journal of Biochemistry & Cell Biology, 44(11), 2060–2064. - PubMed
-
- Bobadilla, J. L. , Macek, M., Jr. , Fine, J. P. , & Farrell, P. M. (2002). Cystic fibrosis: a worldwide analysis of CFTR mutations–correlation with incidence data and application to screening. Human Mutation, 19(6), 575–606. - PubMed
-
- Brena, C. , Chipman, A. D. , Minelli, A. , & Akam, M. (2006). Expression of trunk Hox genes in the centipede Strigamia maritima: sense and anti‐sense transcripts. Evolution & Development, 8(3), 252–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

