Pharmacological Treatment of Vascular Dementia: A Molecular Mechanism Perspective
- PMID: 33532143
- PMCID: PMC7801279
- DOI: 10.14336/AD.2020.0427
Pharmacological Treatment of Vascular Dementia: A Molecular Mechanism Perspective
Abstract
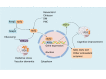
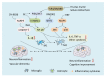
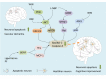
Vascular dementia (VaD) is a neurodegenerative disease, with cognitive dysfunction attributable to cerebrovascular factors. At present, it is the second most frequently occurring type of dementia in older adults (after Alzheimer's disease). The underlying etiology of VaD has not been completely elucidated, which limits its management. Currently, there are no approved standard treatments for VaD. The drugs used in VaD are only suitable for symptomatic treatment and cannot prevent or reduce the occurrence and progression of VaD. This review summarizes the current status of pharmacological treatment for VaD, from the perspective of the molecular mechanisms specified in various pathogenic hypotheses, including oxidative stress, the central cholinergic system, neuroinflammation, neuronal apoptosis, and synaptic plasticity. As VaD is a chronic cerebrovascular disease with multifactorial etiology, combined therapy, targeting multiple pathophysiological factors, may be the future trend in VaD.
Keywords: Vascular dementia; central cholinergic system; neuroinflammation; neuronal apoptosis; oxidative stress; pharmacological treatment; synaptic plasticity.
copyright: © 2021 Kuang et al.
Conflict of interest statement
Declaration of interest The authors declare no conflict of interest.
Figures
References
-
- Wu YT, Fratiglioni L, Matthews FE, Lobo A, Breteler MM, Skoog I, et al. (2016). Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol, 15:116-124. - PubMed
-
- Wolters FJ, Ikram MA (2019). Epidemiology of Vascular Dementia. Arterioscler Thromb Vasc Biol, 39:1542-1549. - PubMed
-
- Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, et al. (2020). Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol, 19:81-92. - PubMed
-
- Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, et al. (2013). Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet, 381:2016-2023. - PubMed
-
- Sachdev PS, Blacker D, Blazer DG, Ganguli M, Jeste DV, Paulsen JS, et al. (2014). Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol, 10:634-642. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
