Activated PKB/GSK-3 β synergizes with PKC- δ signaling in attenuating myocardial ischemia/reperfusion injury via potentiation of NRF2 activity: Therapeutic efficacy of dihydrotanshinone-I
- PMID: 33532181
- PMCID: PMC7838031
- DOI: 10.1016/j.apsb.2020.09.006
Activated PKB/GSK-3 β synergizes with PKC- δ signaling in attenuating myocardial ischemia/reperfusion injury via potentiation of NRF2 activity: Therapeutic efficacy of dihydrotanshinone-I
Abstract
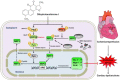
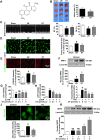
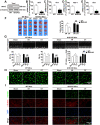
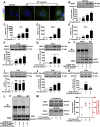
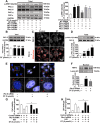
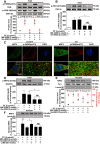
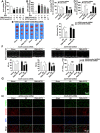
Disrupted redox status primarily contributes to myocardial ischemia/reperfusion injury (MIRI). NRF2, the endogenous antioxidant regulator, might provide therapeutic benefits. Dihydrotanshinone-I (DT) is an active component in Salvia miltiorrhiza with NRF2 induction potency. This study seeks to validate functional links between NRF2 and cardioprotection of DT and to investigate the molecular mechanism particularly emphasizing on NRF2 cytoplasmic/nuclear translocation. DT potently induced NRF2 nuclear accumulation, ameliorating post-reperfusion injuries via redox alterations. Abrogated cardioprotection in NRF2-deficient mice and cardiomyocytes strongly supports NRF2-dependent cardioprotection of DT. Mechanistically, DT phosphorylated NRF2 at Ser40, rendering its nuclear-import by dissociating from KEAP1 and inhibiting degradation. Importantly, we identified PKC-δ-(Thr505) phosphorylation as primary upstream event triggering NRF2-(Ser40) phosphorylation. Knockdown of PKC-δ dramatically retained NRF2 in cytoplasm, convincing its pivotal role in mediating NRF2 nuclear-import. NRF2 activity was further enhanced by activated PKB/GSK-3β signaling via nuclear-export signal blockage independent of PKC-δ activation. By demonstrating independent modulation of PKC-δ and PKB/GSK-3β/Fyn signaling, we highlight the ability of DT to exploit both nuclear import and export regulation of NRF2 in treating reperfusion injury harboring redox homeostasis alterations. Coactivation of PKC and PKB phenocopied cardioprotection of DT in vitro and in vivo, further supporting the potential applicability of this rationale.
Keywords: Cytoplasmic/nuclear translocation; Dihydrotanshinone I; Ischemia/reperfusion injury; NRF2; PKB/GSK-3β/Fyn; PKC-δ; Phosphorylation; Redox homeostasis.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures

References
-
- Levine G.N., Bates E.R., Blankenship J.C., Bailey S.R., Bittl J.A., Cercek B. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: An update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol. 2016;67:1235–1250. - PubMed
-
- Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

