A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy
- PMID: 33532190
- PMCID: PMC7838026
- DOI: 10.1016/j.apsb.2020.07.026
A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy
Abstract
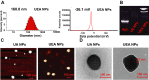
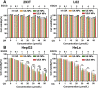
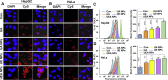
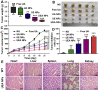
Nanotechnology has emerged as an ideal approach for achieving the efficient chemo agent delivery. However, the potential toxicity and unclear internal metabolism of most nano-carriers was still a major obstacle for the clinical application. Herein, a novel "core‒shell" co-assembly carrier-free nanosystem was constructed based on natural sources of ursolic acid (UA) and polyphenol (EGCG) with the EpCAM-aptamer modification for hepatocellular carcinoma (HCC) synergistic treatment. As the nature products derived from food-plant, UA and EGCG had good anticancer activities and low toxicity. With the simple and "green" method, the nanodrugs had the advantages of good stability, pH-responsive and strong penetration of tumor tissues, which was expected to increase tumor cellular uptake, long circulation and effectively avoid the potential defects of traditional carriers. The nanocomplex exhibited the low cytotoxicity in the normal cells in vitro, good biosafety of organic tissues and efficient tumor accumulation in vivo. Importantly, UA combined with EGCG showed the immunotherapy by activating the innate immunity and acquired immunity resulting in significant synergistic therapeutic effect. The research could provide new ideas for the research and development of self-assembly delivery system in the future, and offer effective intervention strategies for clinical HCC treatment.
Keywords: Aptamer; Co-assembly; EGCG; HCC; Immunotherapy; Nanodrug; Natural product; Synergistic treatment; Ursolic acid.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures




References
-
- Chen S.Z., Cao Q.Q., Wen W., Wang H.Y. Targeted therapy for hepatocellular carcinoma: challenges and opportunities. Canc Lett. 2019;460:1–9. - PubMed
-
- Sia D., Villanueva A., Friedman S.L., Llovet J.M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. - PubMed
-
- Ghosh D., Choudhury S.T., Ghosh S., Mandal A.K., Sarkar S., Ghosh A. Nanocapsulated curcumin: oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chem Biol Interact. 2012;195:206–214. - PubMed
-
- Asghar U., Meyer T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J Hepatol. 2012;56:686–695. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

