Co-therapy with S-adenosylmethionine and nicotinamide riboside improves t-cell survival and function in Arts Syndrome (PRPS1 deficiency)
- PMID: 33532242
- PMCID: PMC7823043
- DOI: 10.1016/j.ymgmr.2021.100709
Co-therapy with S-adenosylmethionine and nicotinamide riboside improves t-cell survival and function in Arts Syndrome (PRPS1 deficiency)
Abstract
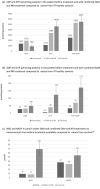
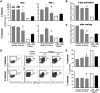
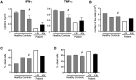
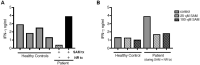
Arts syndrome or phosphoribosyl-pyrophosphate-synthetase-1 (PRPS1) deficiency is caused by loss-of-function mutations in the PRPS1 gene (Xq22.3). PRPS1 is an initial and essential step for the synthesis of the nucleotides of purines, pyrimidines, and nicotinamide. Classically, affected males present with sensorineural hearing loss, optic atrophy, muscular hypotonia, developmental impairment, and recurrent severe respiratory infections early in life. Treatment of a 3-year old boy with S-adenosylmethionine (SAM) replenished erythrocyte purine nucleotides of adenosine and guanosine, while SAM and nicotinamide riboside co-therapy further improved his clinical phenotype as well as T-cell survival and function.
Keywords: 5-phosphoribosyl-1- pyrophosphate, PRPP; Adenosine triphosphate, ATP; Guanosine triphosphate, GTP; NAD phosphate, NADP; Nicotinamide riboside, NR; PRPP synthase, PRPPS; S-adenosylmethionine, SAM; nicotinamide adenine dinucleotide, NAD; phosphoribosyl-pyrophosphate-synthetase-1, PRPS1.
© 2021 The Authors.
Figures
Similar articles
-
S-adenosylmethionine and nicotinamide riboside therapy in Arts syndrome: A case report and literature review.JIMD Rep. 2023 Sep 1;64(6):417-423. doi: 10.1002/jmd2.12395. eCollection 2023 Nov. JIMD Rep. 2023. PMID: 37927483 Free PMC article.
-
The PRPP synthetase spectrum: what does it demonstrate about nucleotide syndromes?Nucleosides Nucleotides Nucleic Acids. 2011 Dec;30(12):1129-39. doi: 10.1080/15257770.2011.591747. Nucleosides Nucleotides Nucleic Acids. 2011. PMID: 22132967 Review.
-
Contribution of Model Organisms to Investigating the Far-Reaching Consequences of PRPP Metabolism on Human Health and Well-Being.Cells. 2022 Jun 13;11(12):1909. doi: 10.3390/cells11121909. Cells. 2022. PMID: 35741038 Free PMC article. Review.
-
Atypical presentation of Arts syndrome due to a novel hemizygous loss-of-function variant in the PRPS1 gene.Mol Genet Metab Rep. 2020 Nov 18;25:100677. doi: 10.1016/j.ymgmr.2020.100677. eCollection 2020 Dec. Mol Genet Metab Rep. 2020. PMID: 33294372 Free PMC article.
-
Arts syndrome is caused by loss-of-function mutations in PRPS1.Am J Hum Genet. 2007 Sep;81(3):507-18. doi: 10.1086/520706. Epub 2007 Aug 3. Am J Hum Genet. 2007. PMID: 17701896 Free PMC article.
Cited by
-
S-adenosylmethionine and nicotinamide riboside therapy in Arts syndrome: A case report and literature review.JIMD Rep. 2023 Sep 1;64(6):417-423. doi: 10.1002/jmd2.12395. eCollection 2023 Nov. JIMD Rep. 2023. PMID: 37927483 Free PMC article.
-
Spectrum of variants associated with inherited retinal dystrophies in Northeast Mexico.BMC Ophthalmol. 2024 Feb 12;24(1):60. doi: 10.1186/s12886-023-03276-7. BMC Ophthalmol. 2024. PMID: 38347443 Free PMC article.
-
Integrated Blood Transcriptome and Multi-Tissue Trace Mineral Analyses of Healthy Stocker Cattle Fed Complexed or Inorganic Trace Mineral Supplement.Animals (Basel). 2024 Jul 26;14(15):2186. doi: 10.3390/ani14152186. Animals (Basel). 2024. PMID: 39123712 Free PMC article.
-
Hypothesis: evidence that the PRS gene products of Saccharomyces cerevisiae support both PRPP synthesis and maintenance of cell wall integrity.Curr Genet. 2024 May 11;70(1):6. doi: 10.1007/s00294-024-01290-w. Curr Genet. 2024. PMID: 38733432 Free PMC article.
-
Nicotinamide Riboside, a Promising Vitamin B3 Derivative for Healthy Aging and Longevity: Current Research and Perspectives.Molecules. 2023 Aug 15;28(16):6078. doi: 10.3390/molecules28166078. Molecules. 2023. PMID: 37630330 Free PMC article. Review.
References
-
- Arts W.F., Loonen M.C., Sengers R.C. X-linked ataxia, weakness, deafness, and loss of vision in early childhood with a fatal course. Ann. Neurol. 1993;33:535–539. - PubMed
-
- Synofzik M., Müller Vom Hagen J., Haack T.B. X-linked Charcot-Marie-Tooth disease, Arts syndrome, and prelingual non-syndromic deafness form a disease continuum: evidence from a family with a novel PRPS1 mutation. Orphanet J Rare Dis. 2014 Feb 14;9:24. doi: 10.1186/1750-1172-9-24. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

