ICS-formoterol reliever versus ICS and short-acting β2-agonist reliever in asthma: a systematic review and meta-analysis
- PMID: 33532465
- PMCID: PMC7836558
- DOI: 10.1183/23120541.00701-2020
ICS-formoterol reliever versus ICS and short-acting β2-agonist reliever in asthma: a systematic review and meta-analysis
Abstract
Background: The Global Initiative for Asthma recommends as-needed inhaled corticosteroid (ICS)-formoterol as an alternative to maintenance ICS plus short-acting β2-agonist (SABA) reliever at step 2 of its stepwise treatment algorithm. Our aim was to assess the efficacy and safety of these two treatment regimens, with a focus on prevention of severe exacerbation.
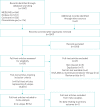
Methods: We performed a systematic review and meta-analysis of all randomised controlled trials (RCTs) comparing as-needed ICS-formoterol with maintenance ICS plus SABA. MEDLINE, Embase, the Cochrane Central Register of Controlled Trials and Clinicaltrials.gov were searched from database inception to 12 December 2019. The primary outcome was time to first severe exacerbation. RCTs were excluded if they used as-needed budesonide-formoterol as part of a maintenance and reliever regimen, or did not report on severe exacerbations. The review is registered with PROSPERO (identifier number CRD42020154680).
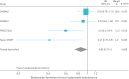
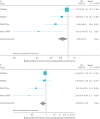
Results: Four RCTs (n=8065 participants) were included in the analysis. As-needed ICS-formoterol was associated with a prolonged time to first severe exacerbation (hazard ratio 0.85, 95% CI 0.73-1.00; p=0.048) and reduced daily ICS dose (mean difference -177.3 μg, 95% CI -182.2--172.4 μg). Asthma symptom control was worse in the as-needed group (Asthma Control Questionnaire-5 mean difference 0.12, 95% CI 0.09-0.14), although this did not meet the minimal clinically important difference of 0.50 units. There was no significant difference in serious adverse events (OR 1.07, 95% CI 0.84-1.36).
Conclusion: As-needed ICS-formoterol offers a therapeutic alternative to maintenance low-dose ICS plus SABA in asthma and may be the preferred option when prevention of severe exacerbation is the primary aim of treatment.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: L. Hatter has nothing to disclose. Conflict of interest: P. Bruce has nothing to disclose. Conflict of interest: I. Braithwaite reports personal fees from Equillium outside the submitted work. Conflict of interest: M. Holliday reports grants from AstraZeneca and the Health Research Council of New Zealand outside the submitted work. Conflict of interest: J. Fingleton reports grants, personal fees and nonfinancial support from AstraZeneca; grants from Genentech; grants, personal fees and nonfinancial support from GlaxoSmithKline; and personal fees and nonfinancial support from Boehringer lngleheim, all outside the submitted work. Conflict of interest: M. Weatherall has nothing to disclose. Conflict of interest: R. Beasley reports grants and personal fees from AstraZeneca, grants from Genentech and GlaxoSmithKline, and personal fees from Theravance and Avillion, outside the submitted work.
Figures

References
-
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2020. Available from: http://ginasthma.com Date last accessed: May 6, 2020.
-
- Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA 2018; 319: 1485–1496. doi: 10.1001/jama.2018.2769 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
