Power of Biocatalysis for Organic Synthesis
- PMID: 33532569
- PMCID: PMC7844857
- DOI: 10.1021/acscentsci.0c01496
Power of Biocatalysis for Organic Synthesis
Abstract
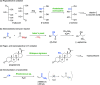
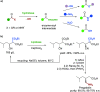
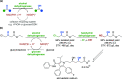
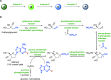
Biocatalysis, using defined enzymes for organic transformations, has become a common tool in organic synthesis, which is also frequently applied in industry. The generally high activity and outstanding stereo-, regio-, and chemoselectivity observed in many biotransformations are the result of a precise control of the reaction in the active site of the biocatalyst. This control is achieved by exact positioning of the reagents relative to each other in a fine-tuned 3D environment, by specific activating interactions between reagents and the protein, and by subtle movements of the catalyst. Enzyme engineering enables one to adapt the catalyst to the desired reaction and process. A well-filled biocatalytic toolbox is ready to be used for various reactions. Providing nonnatural reagents and conditions and evolving biocatalysts enables one to play with the myriad of options for creating novel transformations and thereby opening new, short pathways to desired target molecules. Combining several biocatalysts in one pot to perform several reactions concurrently increases the efficiency of biocatalysis even further.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Wöhler F. Ueber Künstliche Bildung des Harnstoffs. Ann. Phys. 1828, 88, 253–256. 10.1002/andp.18280880206. - DOI
-
- Keen R.The Life and Work of Friedrich Wöhler (1800–1882), 2nd ed.; Nordhausen Bautz: 2005.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

