Heterogeneity in 2,6-Linked Sialic Acids Potentiates Invasion of Breast Cancer Epithelia
- PMID: 33532574
- PMCID: PMC7844859
- DOI: 10.1021/acscentsci.0c00601
Heterogeneity in 2,6-Linked Sialic Acids Potentiates Invasion of Breast Cancer Epithelia
Abstract
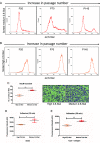
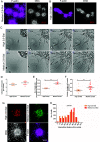
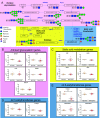
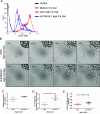
Heterogeneity in phenotypes of malignantly transformed cells and aberrant glycan expression on their surface are two prominent hallmarks of cancers that have hitherto not been linked to each other. In this paper, we identify differential levels of a specific glycan linkage: α2,6-linked sialic acids within breast cancer cells in vivo and in culture. Upon sorting out two populations with moderate, and relatively higher, cell surface α2,6-linked sialic acid levels from the triple-negative breast cancer cell line MDA-MB-231, both populations (denoted as medium and high 2,6-Sial cells, respectively) stably retained their levels in early passages. Upon continuous culturing, medium 2,6-Sial cells recapitulated the heterogeneity of the unsorted line whereas high 2,6-Sial cells showed no such tendency. Compared with high 2,6-Sial cells, the medium 2,6-Sial counterparts showed greater adhesion to reconstituted extracellular matrices (ECMs) and invaded faster as single cells. The level of α2,6-linked sialic acids in the two sublines was found to be consistent with the expression of a specific glycosyl transferase, ST6GAL1. Stably knocking down ST6GAL1 in the high 2,6-Sial cells enhanced their invasiveness. When cultured together, medium 2,6-Sial cells differentially migrated to the edge of growing tumoroid-like cocultures, whereas high 2,6-Sial cells formed the central bulk. Multiscale simulations in a Cellular Potts model-based computational environment calibrated to our experimental findings suggest that differential levels of cell-ECM adhesion, likely regulated by α2,6-linked sialic acids, facilitate niches of highly invasive cells to efficiently migrate centrifugally as the invasive front of a malignant breast tumor.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Verhaegh W.; et al. Heterogeneity in signaling pathway activity within primary breast cancer and between primary and metastases. J. Clin. Oncol. 2019, 37 (15s), 589–589. 10.1200/JCO.2019.37.15_suppl.589. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

