IDentif.AI: Rapidly optimizing combination therapy design against severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Cov-2) with digital drug development
- PMID: 33532594
- PMCID: PMC7823122
- DOI: 10.1002/btm2.10196
IDentif.AI: Rapidly optimizing combination therapy design against severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Cov-2) with digital drug development
Abstract
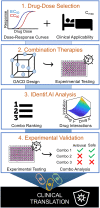
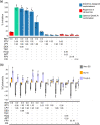
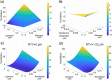
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to multiple drug repurposing clinical trials that have yielded largely uncertain outcomes. To overcome this challenge, we used IDentif.AI, a platform that pairs experimental validation with artificial intelligence (AI) and digital drug development to rapidly pinpoint unpredictable drug interactions and optimize infectious disease combination therapy design with clinically relevant dosages. IDentif.AI was paired with a 12-drug candidate therapy set representing over 530,000 drug combinations against the SARS-CoV-2 live virus collected from a patient sample. IDentif.AI pinpointed the optimal combination as remdesivir, ritonavir, and lopinavir, which was experimentally validated to mediate a 6.5-fold enhanced efficacy over remdesivir alone. Additionally, it showed hydroxychloroquine and azithromycin to be relatively ineffective. The study was completed within 2 weeks, with a three-order of magnitude reduction in the number of tests needed. IDentif.AI independently mirrored clinical trial outcomes to date without any data from these trials. The robustness of this digital drug development approach paired with in vitro experimentation and AI-driven optimization suggests that IDentif.AI may be clinically actionable toward current and future outbreaks.
Keywords: COVID‐19; SARS‐CoV‐2; artificial intelligence; combinatory treatment; digital medicine; drug development; drug interactions.
© 2020 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of The American Institute of Chemical Engineers.
Conflict of interest statement
A. B., T. K., L. H., X. D., E. K‐.H. C., and D. H. are co‐inventors or previously filed pending patents on artificial intelligence‐based therapy development. T. K. E. K.‐H. C., and D. H. are shareholders of KYAN Therapeutics, which has licensed intellectual property pertaining to AI‐based drug development. No intellectual property rights from this reported work are being pursued.
Figures
Similar articles
-
Addressing antimicrobial resistance with the IDentif.AI platform: Rapidly optimizing clinically actionable combination therapy regimens against nontuberculous mycobacteria.Theranostics. 2022 Sep 25;12(16):6848-6864. doi: 10.7150/thno.73078. eCollection 2022. Theranostics. 2022. PMID: 36276648 Free PMC article.
-
The IDentif.AI-x pandemic readiness platform: Rapid prioritization of optimized COVID-19 combination therapy regimens.NPJ Digit Med. 2022 Jun 30;5(1):83. doi: 10.1038/s41746-022-00627-4. NPJ Digit Med. 2022. PMID: 35773329 Free PMC article.
-
IDentif.AI-Omicron: Harnessing an AI-Derived and Disease-Agnostic Platform to Pinpoint Combinatorial Therapies for Clinically Actionable Anti-SARS-CoV-2 Intervention.ACS Nano. 2022 Sep 27;16(9):15141-15154. doi: 10.1021/acsnano.2c06366. Epub 2022 Aug 17. ACS Nano. 2022. PMID: 35977379
-
Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2.Biomed Pharmacother. 2020 Nov;131:110668. doi: 10.1016/j.biopha.2020.110668. Epub 2020 Aug 24. Biomed Pharmacother. 2020. PMID: 32861965 Free PMC article. Review.
-
A Review of the Preclinical and Clinical Efficacy of Remdesivir, Hydroxychloroquine, and Lopinavir-Ritonavir Treatments against COVID-19.SLAS Discov. 2020 Dec;25(10):1108-1122. doi: 10.1177/2472555220958385. Epub 2020 Sep 17. SLAS Discov. 2020. PMID: 32942923 Free PMC article. Review.
Cited by
-
Splice-switch oligonucleotide-based combinatorial platform prioritizes synthetic lethal targets CHK1 and BRD4 against MYC-driven hepatocellular carcinoma.Bioeng Transl Med. 2022 Sep 3;8(1):e10363. doi: 10.1002/btm2.10363. eCollection 2023 Jan. Bioeng Transl Med. 2022. PMID: 36684069 Free PMC article.
-
Addressing antimicrobial resistance with the IDentif.AI platform: Rapidly optimizing clinically actionable combination therapy regimens against nontuberculous mycobacteria.Theranostics. 2022 Sep 25;12(16):6848-6864. doi: 10.7150/thno.73078. eCollection 2022. Theranostics. 2022. PMID: 36276648 Free PMC article.
-
Identifying Structural Features of Nucleotide Analogues to Overcome SARS-CoV-2 Exonuclease Activity.Viruses. 2022 Jun 28;14(7):1413. doi: 10.3390/v14071413. Viruses. 2022. PMID: 35891393 Free PMC article.
-
Flash optimization of drug combinations for Acinetobacter baumannii with IDentif.AI-AMR.NPJ Antimicrob Resist. 2025 Feb 21;3(1):12. doi: 10.1038/s44259-025-00079-2. NPJ Antimicrob Resist. 2025. PMID: 39984645 Free PMC article.
-
N-of-1 medicine.Singapore Med J. 2024 Mar 1;65(3):167-175. doi: 10.4103/singaporemedj.SMJ-2023-243. Epub 2024 Mar 26. Singapore Med J. 2024. PMID: 38527301 Free PMC article.
References
-
- NIH . NIH clinical trial shows Remdesivir accelerates recovery from advanced COVID‐19. NIH. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-r.... Accessed 29 Apr, 2020
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

