Statistical fundamentals on cancer research for clinicians: Working with your statisticians
- PMID: 33532634
- PMCID: PMC7829109
- DOI: 10.1016/j.ctro.2021.01.006
Statistical fundamentals on cancer research for clinicians: Working with your statisticians
Abstract
Purpose: To facilitate understanding statistical principles and methods for clinicians involved in cancer research.
Methods: An overview of study design is provided on cancer research for both observational and clinical trials addressing study objectives and endpoints, superiority tests, non-inferiority and equivalence design, and sample size calculation. The principles of statistical models and tests including contemporary standard methods of analysis and evaluation are discussed. Finally, some statistical pitfalls frequently evident in clinical and translational studies in cancer are discussed.
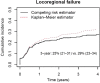
Results: We emphasize the practical aspects of study design (superiority vs non-inferiority vs equivalence study) and assumptions underpinning power calculations and sample size estimation. The differences between relative risk, odds ratio, and hazard ratio, understanding outcome endpoints, purposes of interim analysis, and statistical modeling to minimize confounding effects and bias are also discussed.
Conclusion: Proper design and correctly constructed statistical models are critical for the success of cancer research studies. Most statistical inaccuracies can be minimized by following essential statistical principles and guidelines to improve quality in research studies.
Keywords: Cancer; Clinical research; Data analysis; Statistical models; Statistics; Study design.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Multiple-arm superiority and non-inferiority designs with various endpoints.Pharm Stat. 2007 Jan-Mar;6(1):43-52. doi: 10.1002/pst.242. Pharm Stat. 2007. PMID: 17323311
-
Clinical trials of antibacterial agents: a practical guide to design and analysis. Statisticians in the Pharmaceutical Industry Working Party.J Antimicrob Chemother. 1998 Apr;41(4):467-80. doi: 10.1093/jac/41.4.467. J Antimicrob Chemother. 1998. PMID: 9598778
-
Active-control clinical trials to establish equivalence or noninferiority: methodological and statistical concepts linked to quality.Am Heart J. 2003 Sep;146(3):398-403. doi: 10.1016/S0002-8703(03)00324-7. Am Heart J. 2003. PMID: 12947355
-
Non-inferiority trials in cardiology: what clinicians need to know.Heart. 2020 Jan;106(2):99-104. doi: 10.1136/heartjnl-2019-315772. Epub 2019 Oct 31. Heart. 2020. PMID: 31672779 Free PMC article. Review.
-
[Methodological and statistical aspects of equivalence and non inferiority trials].Rev Epidemiol Sante Publique. 2008 Aug;56(4):267-77. doi: 10.1016/j.respe.2008.05.027. Epub 2008 Aug 13. Rev Epidemiol Sante Publique. 2008. PMID: 18703296 Review. French.
Cited by
-
The Clinicopathological Characteristics of Young-Onset Versus Adult-Onset Colorectal Cancer: A Tertiary Hospital-Based Study.Malays J Med Sci. 2024 Feb;31(1):200-211. doi: 10.21315/mjms2024.31.1.17. Epub 2024 Feb 28. Malays J Med Sci. 2024. PMID: 38456100 Free PMC article.
-
Increasing Power in Phase III Oncology Trials With Multivariable Regression: An Empirical Assessment of 535 Primary End Point Analyses.JCO Clin Cancer Inform. 2024 Sep;8:e2400102. doi: 10.1200/CCI.24.00102. JCO Clin Cancer Inform. 2024. PMID: 39213473
-
Cytotoxic Effects of Common Irrigation Solutions on Chondrosarcoma and Giant Cell Tumors of Bone.J Bone Joint Surg Am. 2022 Dec 21;104(24):2153-2159. doi: 10.2106/JBJS.22.00404. Epub 2022 Oct 27. J Bone Joint Surg Am. 2022. PMID: 36367764 Free PMC article.
-
Radiomics and machine learning for predicting metachronous liver metastasis in rectal cancer.World J Gastrointest Oncol. 2025 Apr 15;17(4):102324. doi: 10.4251/wjgo.v17.i4.102324. World J Gastrointest Oncol. 2025. PMID: 40235892 Free PMC article.
-
Impact of the COVID-19 pandemic on lung cancer diagnosis in northern Poland-addressing the COVID-19 debt.PLoS One. 2024 Dec 27;19(12):e0316261. doi: 10.1371/journal.pone.0316261. eCollection 2024. PLoS One. 2024. PMID: 39729487 Free PMC article.
References
-
- Sacca L. The uncontrolled clinical trial: scientific, ethical, and practical reasons for being. Intern Emerg Med. 2010;5(3):201–204. - PubMed
-
- Verweij J., Hendriks H.R., Zwierzina H. Cancer drug development F. Innovation in oncology clinical trial design. Cancer Treat Rev. 2019;74:15–20. - PubMed
-
- Sherman R.E., Anderson S.A., Dal Pan G.J. Real-world evidence – What is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources