Pregnancy-Associated Venous Thromboembolism: Insights from GARFIELD-VTE
- PMID: 33532693
- PMCID: PMC7840428
- DOI: 10.1055/s-0040-1722611
Pregnancy-Associated Venous Thromboembolism: Insights from GARFIELD-VTE
Abstract
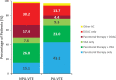
Introduction The risk of venous thromboembolism (VTE) increases during pregnancy and the puerperium such that VTE is a leading cause of maternal mortality. Methods We describe the clinical characteristics, diagnostic strategies, treatment patterns, and outcomes of women with pregnancy-associated VTE (PA-VTE) enrolled in the Global Anticoagulant Registry in the FIELD (GARFIELD)-VTE. Women of childbearing age (<45 years) were stratified into those with PA-VTE ( n = 183), which included pregnant patients and those within the puerperium, and those with nonpregnancy associated VTE (NPA-VTE; n = 1,187). Patients with PA-VTE were not stratified based upon the stage of pregnancy or puerperium. Results Women with PA-VTE were younger (30.5 vs. 34.8 years), less likely to have pulmonary embolism (PE) (19.7 vs. 32.3%) and more likely to have left-sided deep vein thrombosis (DVT) (73.9 vs. 54.8%) compared with those with NPA-VTE. The most common risk factors in PA-VTE patients were hospitalization (10.4%), previous surgery (10.4%), and family history of VTE (9.3%). DVT was typically diagnosed by compression ultrasonography (98.7%) and PE by chest computed tomography (75.0%). PA-VTE patients more often received parenteral (43.2 vs. 15.1%) or vitamin K antagonists (VKA) (9.3 vs. 7.6%) therapy alone. NPA-VTE patients more often received a DOAC alone (30.2 vs. 13.7%). The risk (hazard ratio [95% confidence interval]) of all-cause mortality (0.59 [0.18-1.98]), recurrent VTE (0.82 [0.34-1.94]), and major bleeding (1.13 [0.33-3.90]) were comparable between PA-VTE and NPA-VTE patients. Uterine bleeding was the most common complication in both groups. Conclusion VKAs or DOACs are widely used for treatment of PA-VTE despite limited evidence for their use in this population. Rates of clinical outcomes were comparable between groups.
Keywords: deep vein thrombosis; pregnancy; pulmonary embolism; registry; venous thromboembolism.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. ( https://creativecommons.org/licenses/by/4.0/ ).
Conflict of interest statement
Conflict of Interest C.J.-S. received consultancy fees from Bayer, Pfizer, Actelion, Boehringer, and Meranini. P.M. received personal fees from Bayer Pharma AG. R.D.L. received research grants and personal fees from Bristol-Myers Squibb and Pfizer, personal fees from Boehringer Ingelheim and Bayer AG, and research grants from Amgen Inc, GlaxoSmithKline, Medtronic PLC, and Sanofi Aventis outside the submitted work. A.G.G.T. received personal fees from Bayer Pharma AG and Janssen. J.I.W. received research support from Canadian Institutes of Health Research, Heart and Stroke Foundation, and the Canadian Fund for Innovation; honoraria from Bayer Pharma AG, Boehringer-Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Ionis, Janssen, Merck, Portola, Pfizer, Servier, Novartis, Anthos, and Tetherex. S.H. received honoraria from Bayer Pharma AG, Bristol Myers Squibb, Daiichi-Sankyo, Pfizer, and Portola. W.A. received honoraria from Boehringer Ingelheim, Bayer Pharma AG, Bristol Myers Squibb, Pfizer, Daiichi-Sankyo, Portola, Aspen, Sanofi. S.G. got research funding from Ono, Bristol Myers Squibb, Sanofi, and Pfizer; personal fees from Thrombosis Research Institute and the American Heart Association. S.Z.G. received research support from Bayer Pharma AG, Boehringer-Ingelheim, BMS, BTG EKOS, Daiichi-Sankyo, Janssen, NHLBI, Thrombosis Research Institute; consultancy fees from Bayer Pharma AG, Boehringer-Ingelheim. J.D.N. received honoraria from Bayer Pharma AG, Boehringer-Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Leo Pharma, and Pfizer. S.S. received speaker fees from Bayer Pharma AG, Boehringer-Ingelheim, Bristol Meyer Squibb, Daiichi-Sankyo, Sanofi Aventis, and Pfizer; consultancy fees from Bayer Pharma AG, Boehringer-Ingelheim, Daiichi-Sankyo, Sanofi Aventis, Aspen, and Pfizer. H.B. received honoraria from Bayer Pharma (Switzerland) AG. L.G.M. received grants and personal fees from Bayer Pharma AG, Boehringer-Ingelheim, Pfizer, and Daiichi-Sankyo. P.P. received personal fees from Bayer Pharma AG, Pfizer, Daiichi-Sankyo, and Sanofi. A.K.K. received research grants from Bayer Pharma AG; personal fees from Bayer Pharma AG, Sanofi S.A., Janssen Pharma, Verseon, and Pfizer. D.R. , P.A., A.E.F., and G.K. declare that they have no conflict of interest in the research.
Figures


References
-
- van Es N, Coppens M, Schulman S, Middeldorp S, Büller H R. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968–1975. - PubMed
-
- Heit J A, Kobbervig C E, James A H, Petterson T M, Bailey K R, Melton L J., III Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143(10):697–706. - PubMed
-
- Pomp E R, Lenselink A M, Rosendaal F R, Doggen C J. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6(04):632–637. - PubMed
-
- Jerjes-Sánchez C, García-Sosa A. 2015. Thrombolysis in Special Situations; pp. 211–227.
-
- Marik P E, Plante L A. Venous thromboembolic disease and pregnancy. N Engl J Med. 2008;359(19):2025–2033. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

